Research Article - (2020) Volume 11, Issue 1
Dipeptidyl peptidase-4 (DPP-4) inhibitors constitute an innovative class of oral agents for the treatment of Type 2 Diabetes Mellitus (T2DM). DPP-4 inhibitors increase glucagon-like peptide-1 (GLP-1) availability and correct the “incretin defect” seen in T2DM patients. Peptides derived from collagen have been reported to have DPP-4 inhibitory properties. A double blind randomized trial has been conducted to evaluate the effectiveness of Collagen peptides (CPT) as nutritional supplement in subjects with T2DM. Resistant dextrin (RD), a non-digestible dietary polymer, has been used as active comparator in this study. The clinical study was conducted over a total duration of 12 weeks of treatment period. The study was conducted on 66 enrolled subjects randomized in a 2:2:1:1 ratio as a four arm clinical study design. The subjects consumed either CPT (2.5/5 g) or resistant dextrin (2.5/5 g) for 90 days. The results showed that the consumption of 5 g CPT resulted in significant reduction in fasting blood glucose (FBG) and Glycosylated Haemoglobin (HbA1c) in three months study period in subjects. Insulin sensitivity measured in as HOMA IR has also been improved significantly in the group. Thus this study demonstrates the potential role of CPT as add on nutritional supplement for the management of T2DM.
For more detailed information visit the following sites:
https://marmarisdentalcenter.com
https://dentalclinicmarmaris.com
https://marmarisdentals.com
Collagen peptide; DPP-4 inhibition; Type 2 Diabetes Mellitus; Nutritional supplement
Diabetes mellitus represents a series of metabolic conditions associated with hyperglycemia and caused by defects in insulin secretion and/or insulin action. People with diabetes either do not produce enough insulin (Type 1 diabetes) or cannot use insulin properly (Type 2 diabetes). Type 2 diabetes mellitus is a complex endocrine and metabolic disorder, which can lead to chronically high blood sugar levels, causing several symptoms and potentially leading to serious complications. Normally two incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 (GLP-1) are released during a meal and can stimulate insulin secretion from β-cells [1]. However, GIP and GLP-1 are rapidly degraded after secretion by the action of DPP-4, resulting in loss of their insulinotropic activities [2]. Therefore, the use of DPP- 4 inhibitors for elongation of the half-life of incretins, especially GLP-1, is a therapeutic approach for managing T2DM [3]. The ubiquitously expressed enzyme DPP-4 (EC 3.4.14.5) modulates the biological activity of circulating peptide hormones by specifically cleaving the two N-terminal amino acids X-Pro and X-Ala [4], and also metabolizes insulinotropic hormone glucagon-like peptide-1 (GLP-1). Hence DPP-4 inhibitors that protect active GLP-1 from being cleaved by DPP-4 can be used to control postprandial glycemia in T2DM [5,6].
Fish collagen peptide, derived from the skin, bones and scales is used as a functional food or dietary supplement as they are digested and absorbed by the body quickly. Oral Administration of Collagen Hydrolysates Improves Glucose Tolerance in Normal Mice through GLP-1-Dependent and GLP-1-Independent Mechanisms [7]. The polypeptide chains of collagen molecules are composed of numerous repeats of a tripeptide unit, Gly-X-Y, where X and Y are generally proline and hydroxyproline. The Gly-Pro-Hyp tripeptide (10.5%) is the most highly represented of the various tripeptide units; the other tripeptides include Gly-Pro-Ala, Gly- Ala-Hyp and Gly-Leu-Hyp (3.4–5.5%) [8] of which Gly-Pro-Ala is a known DPP- 4 inhibitor [9]. The DPP-4 inhibition property of collagen peptide has great relevance as a natural source for management of T2DM through incretin effect. There are several in vitro [10-12] and studies in animal models [13-15] and human clinical trials [16-18] on hypoglycaemic effect of collagen derived peptides, by making use of its ability to inhibit DPP-4. The US patent by Sugihara et al. [19] describes the potential role of peptides derived from collagen as the therapeutic or preventive agent for diabetes. Collagen peptide mixture containing peptides selected from the group consisting of tri-peptides and dipeptides or chemically modified substances as described in the patent document thereof, have DPP-4 inhibitory activity and/or GLP-1 secretion activity.
The activity of these bioactive peptides is based on their inherent amino acid composition and length of the sequence. As, there is an increasing attention toward natural, safe, food-derived peptide inhibitors without any side effects in the treatment of T2DM, research in the field and clinical studies are very necessary to validate and confirm the efficacy and bioavailability of protein derived peptides in humans. The objective of the present study is to evaluate the comparative efficacy, safety and tolerability of Collagen Peptide vs. Resistant Dextrin as a neutraceutical therapy in the management of T2DM in adults and to determine the most optimal dose of Collagen as a neutraceutical therapy in the management of T2DM in adults. Resistant dextrin (RD), a soluble dietary fiber, is an indigestible glucose polysaccharide, rich in α-1,2 or α-1,3 linkages, formed by the hydrolysis of starch (wheat/maize). RD is suitable for diabetic patients as it absorbs glucose and inhibits the activity of amylase thus delaying the degradation of starch and absorption of glucose, and thereby inhibiting the increase of blood glucose after meals. RD has been classified as FOSHU (Foods for Special Health Uses) in Japan and GRAS (Generally Recognized as Safe) by the Food and Drug Administration in the United States [20].
The reduction in elevated blood glucose levels, reduction in insulin resistance and reduction in the content of glycosylated hemoglobin and overall improvement in quality of life are assessed in the study.
Investigational products
Fish collagen peptide (FCP) is the product of Nitta Gelatin India Limited and the comparator Resistant dextrin (Fibersol®) is purchased from M/s. Brenntag Ingredients.
Study design
The study was designed as a double blind, comparative, randomized, active controlled, four arm clinical study. A double-blind, placebocontrolled clinical trial is one in which neither the participants nor the experimenters know who is receiving a particular treatment. Thus, when such study is ideally performed, it produces knowledge untainted by bias. Randomized controlled trials (RCTs) are quantitative, comparative, controlled experiments in which two or more interventions are studied in a series of individuals who receive them in random order. Double blind randomized controlled trials (DBRCT) is one of the simplest and most powerful tools in clinical research as it provides the most reliable evidence on the effectiveness of interventions by preventing the selection bias and insuring against the accidental bias. It produces comparable groups and eliminates the source of bias in treatment assignments. Here, in the study the research participants are thus assigned by chance, rather than by choice, to either the experimental groups or to the control groups. The study was commenced after approval from Universal Ethical committee, India, which is a CDSCO registered Ethics Committee and after obtaining written informed consent from all subjects.
All of the study subjects (male and female) were adults between the ages of 21 and 50 years (both ages inclusive) with known history/diagnosis of diabetes mellitus (type II). The selected subjects were on standard therapy of Sitagliptin/Vildagliptin for management of diabetes mellitus (type II). Thus ethically the subjects were not deprived of a hypoglycemic agent. The subjects had no known history or diagnosis of complications associated with diabetes mellitus (type II). It was also confirmed that the selected subjects were non-alcoholic, non-smokers and non-users of tobacco. The Body Mass Index of all the selected subjects were between 18 to 32 kg/m2, (both values inclusive). All the subjects were with normal presentations during physical examination and laboratory investigations-complete blood count and serum biochemistry. The vegetarians or vegans who object consumption of animal origin products and those were on herbal, ayurvedic or homeopathic medications for the control of diabetes mellitus were not enrolled in the study. Patients with co morbidity of diseases of chronic nature with concurrent medications were also not enrolled. Anyone who have undergone major surgical procedure 4 weeks prior to randomization or who are on steroids, hormone therapy, anti-depressants, anti-psychotics were excluded from the study. Patients who are known/reported to be pregnant, lactating, planning a pregnancy or unwilling to practice double barrier contraceptive method were excluded from the study. Finally people who are mentally unable to comprehend the responsibilities and adhere to the stipulations of the protocol and those who are in the opinion of the investigator as deemed unfit to participate in the study were also excluded from the study.
A sample size of 66 subjects of male and female who met the study criteria were enrolled in the study in a double blinded fashion into four treatment arms in a 2:2:1:1 ratio. Randomization was performed using a random number table by computer program. In order to accommodate non sampling errors such as nonresponsive or drop outs, an additional 10% of subjects were considered in the study. The details of the study design are given in Figure 1. Fish Collagen Peptide (either 2.5 g or 5 g) was evaluated against an Active control- Resistant Dextrin (same dosages), in a double blinded fashion. The investigational product and the Active Control had been administered orally once a day by dissolving 5 g/2.5 g product in 200 mL luke warm water or milk. As the Active control is therapeutically effective it will justify that all subjects participated in the trial was supplemented with a nutritional supplement instead of using a placebo with no effect.
The design was double blinded and hence the study was unbiased enabling efficient capturing of the effectiveness of collagen over the active comparator in reduction of blood glucose levels and overall improvement in quality of life.
The Four Arm Clinical study, was designed with 40 subjects on CPT arms (Treatment Arm I & Treatment Arm II) and 20 subjects on RD arms (Treatment Arm III & Treatment Arm IV) (Figure 1). The randomization schedule was based on SAS generated randomization schedule. All subjects were enrolled either of the treatment arms using double blind code. The study was conducted over a period of 12 weeks with 5 days of run-in period between screening and enrollment with a window period of ± 2 days. The response to treatment was evaluated by improvements in clinical, laboratory and subjective assessments.
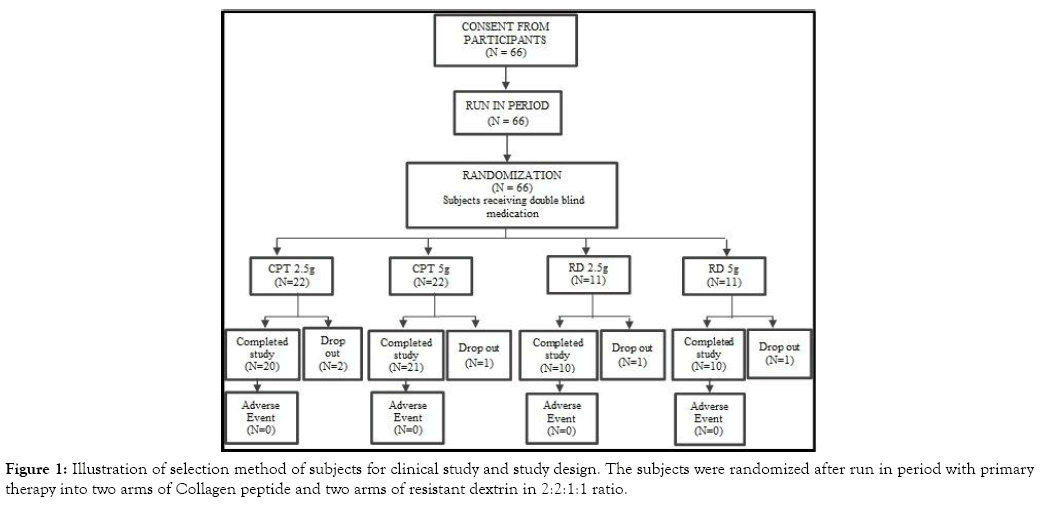
Figure 1: Illustration of selection method of subjects for clinical study and study design. The subjects were randomized after run in period with primary therapy into two arms of Collagen peptide and two arms of resistant dextrin in 2:2:1:1 ratio.
Primary parameters assessed in the study were, Fasting blood glucose levels (FBG), Glycosylated Haemoglobin percentage (HbA1c), Homeostatic Model Assessment of Insulin Resistance (HOMA IR), and Fasting Lipid Profile. A subjective assessment parameter namely Quality of Life Questionnaire (QoL) score was also recorded. The baseline measurements and changes in the subsequent visits are measured with a suitable scale of measurement. In particular, the variables related to laboratory investigations were measured as interval/ratio scale, while QoL score is measured at 5-point Likert scale (ordinal level) from Visit-1 through Visit-5. The sum of the scores of all 15 questions from QoL Questionnaire was considered as a ratio scale variable. The demographic and baseline characteristics are recorded in Table 1.
| Variable | Demographic Data | Baseline Data |
|---|---|---|
| Male | 27 | |
| Female | 33 | |
| Height, Unit: cm | FBG, Unit: mg/dL | |
| Average | 155.68 | 213 |
| Standard deviation | 0.08 | 44.4 |
| Maximum | 165.25 | 309.75 |
| Minimum | 146.00 | 154.5 |
| Weight, Unit: Kg | HbA1c, Unit: % | |
| Average | 64.52 | 8.2 |
| Standard deviation | 12.18 | 0.65 |
| Maximum | 84.35 | 9.575 |
| Minimum | 45.83 | 6.95 |
| BMI, Unit: Kg/m2 | HOMA-IR, Units | |
| Average | 27.40 | 8.5 |
| Standard deviation | 3.67 | 4.3 |
| Maximum | 32.03 | 17.8 |
| Minimum | 19.25 | 3.0 |
Table 1: Demographic and Baseline characteristics.
Improvement metrics were assessed every 3 weeks. For improvement metrics, Fasting Blood glucose, HbA1c and Quality of life questionnaire were assessed. The study treatment was completed after treatment duration of 12 weeks for each enrolled subject. During the end of the study visit, FBG, HbA1c, HOMA-IR, Complete Blood Count and Serum Biochemistry were assessed, along with QoL questionnaire. There was no change in the study procedures or conducting after commencement of the study and hence no analysis has been planned or conducted.
Statistical analysis
Statistical tests are carried out at 5% level of significance with a two-sided hypothesis or one-sided hypothesis, which is depending upon the requirement or objective. Descriptive measures such as mean, standard deviation and proportions are used to model the basic characteristics of the subject/sample. Similarly, parametric tests such as t-test, F-test, and ANOVA are carried out to study continuous type variables such as age and height/weight. The statistical software SAS V-9.1.3 is used to perform both parametric and non-parametric tests and for the generation of tables, graphs and reports. Change from baseline in study specific parameters was analysed using an Analysis of Variance (ANOVA) method with treatment group as fixed factors. McNemar’s test was used to compare between baseline and end of the treatment weeks.
Primary objective of this clinical study was to confirm the efficacy, safety and tolerability of two doses of CPT in comparison with two doses of RD as an add-on nutritional therapy for the management of T2DM in adult subjects. Eighty three subjects were screened and 66 subjects were enrolled into this clinical study. Of this, 5 subjects were lost to follow up at Visit 3. The data of the first 60 subjects to complete the study was considered for statistical analysis. Primary objectives of the study were confirmed by analyzing blood parameters such as Fasting Blood Glucose, HbA1c and Insulin Resistance.
At the end of the study period mean FBG for those treated with 2.5 g of CPT decreased from 229 ± 75.2 mg/dL to 194 ± 60.4 mg/dL (p>0.05) and for those treated with g CPT the FBG decreased from 188 ± 28.6 mg/dL to 110.10 ± 15.4 mg/dL (p<0.05). Whereas, the mean fasting blood glucose for those treated with 2.5 g of RD decreased from 213 ± 33.4 to 203 ± 54.8 mg/dL (p>0.05) and the FBG decreased from 223 ± 40.4 mg/dL to 165 ± 34.5 mg/dL (p>0.05) for those treated with 5 g of RD, Figure 2. FBG of 5 g CPT treated group showed a significant reduction of 41% in FBS at V5 (end of the study) when compared to V0 (initial visit).
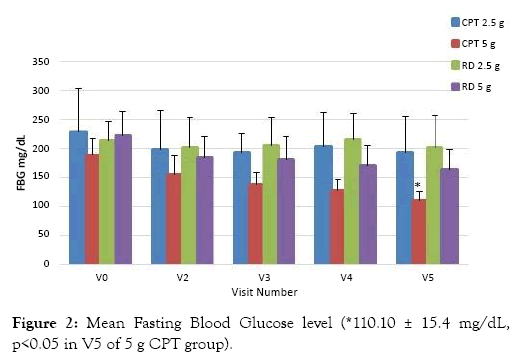
Figure 2: Mean Fasting Blood Glucose level (*110.10 ± 15.4 mg/dL, p<0.05 in V5 of 5 g CPT group).
The results of HbA1c data analysed at the end of the study confirmed that the mean HbA1c level of the 2.5 g CPT treated group decreased from 8.3 ± 1.4 to 7.0 ± 1.2 (p>0.05) and that of 5 g CPT treated group decreased from 8.1 ± 0.6 to 5.9 ± 0.5 (p<0.05) respectively. Whereas, the mean HbA1c for those treated with 2.5 g of RD decreased from 8.0 ± 0.2 to 7.2 ± 0.2 (p>0.05) and for those treated with 5 g RD the HbA1c level decreased from 8.4 ± 0.4 to 7.0 ± 0.6 (p>0.05), Figure 3. There was a significant reduction of 27% in the HbA1c level at V5 when compared to V0 in the 5g CPT supplemented group.
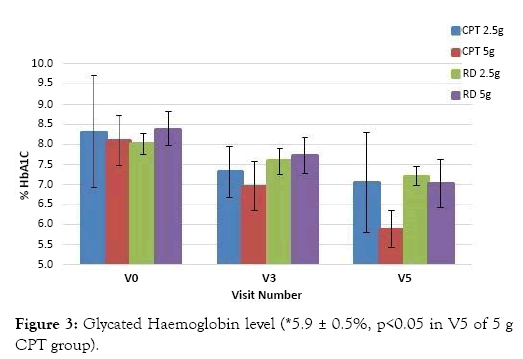
Figure 3: Glycated Haemoglobin level (*5.9 ± 0.5%, p<0.05 in V5 of 5 g CPT group).
The end point data analysis confirms that the average HOMA–IR value decreased from 10.0 ± 5.5 to 8.2 ± 4.0, (p>0.05) and from 7.9 ± 4.1 to 4.7 ± 2.3, (p<0.05) for those treated with 2.5 g CPT and 5 g CPT respectively. Whereas, the the average value decreased from 6.6 ± 2.7 to 6.3 ± 2.3, p>0.05 in the 2.5 g RD group and the average value decreased from 9.4 ± 5.0 to 6.8 ± 2.8, p>0.05 in the 5 g RD group. The mean reduction in HOMA IR for those treated with 2.5 g and 5 g RD was 4.7% and 28% respectively and those treated with 2.5 g and 5 g CPT was 19% and 41% respectively. The data showing the reduction in HOMA-IR is represented in Figure 4.
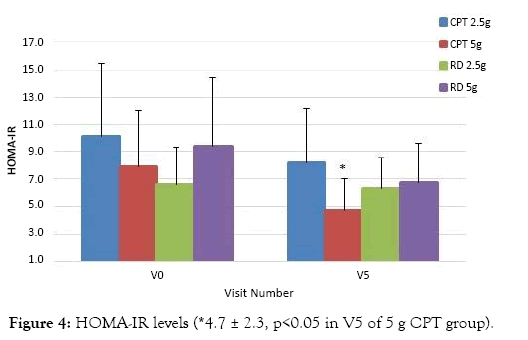
Figure 4: HOMA-IR levels (*4.7 ± 2.3, p<0.05 in V5 of 5 g CPT group).
The data of Quality of Life (QoL) analysis confirms that there was average 22% improvement in the QoL for those treated with 2.5 g CPT with an average increase from 51 ± 7.6 to 62 ± 5.4 (p>0.05). There was an average 39% improvement in QoL for those treated with 5 g CPT with an average increase from 48 ± 4.0 to 67 ± 4.4 in QoL score in V5 (p<0.05). Whereas, only an average QoL improvement of 6% and 16% was observed for those treated with 2.5 g and 5 g RD respectively. The average QoL score for those treated with 2.5 g RD increased from 52 ± 5.8 to 55 ± 6.5 (p>0.05) and for those treated with 5 g of RD the QoL score increased from 53 ± 6.3 to 61 ± 4.2 (p>0.05) in V5. The QoL score data is represented in Figure 5.
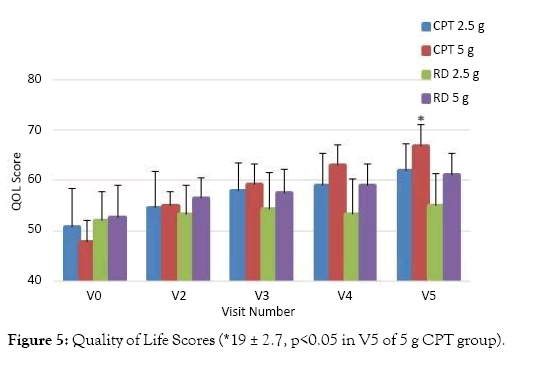
Figure 5: Quality of Life Scores (*19 ± 2.7, p<0.05 in V5 of 5 g CPT group).
Biochemical evaluations
Secondary objectives of the study were to verify the efficacy of the investigational product on lowering total cholesterol levels and also improving the quality of life of the subject. The final data and the baseline values are compared to substantiate the efficacy of secondary objectives in both the CPT groups. As part of safety assessment, laboratory analysis for the various other biochemical parameters were also performed. The results of the Visit 1 and Visit 5 are shown in Table 2.
| Parameters | Unit | 2.5 g CPT | 2.5 g RD | 5.0 g CPT | 5.0 g RD | ||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 5 | Visit 1 | Visit 5 | Visit 1 | Visit 5 | Visit 1 | Visit 5 | ||
| Serum Biochemistry | |||||||||
| Urea | mg/dL | 22.8 ± 6.0 | 21.0 ± 4.9 | 21.6 ± 8.4 | 19.2 ± 5.5 | 19.6 ± 5.5 | 19.5 ± 5.8 | 24.0 ± 6.6 | 24.7 ± 4.9 |
| Serum Creatinine | mg/dL | 0.9 ± 0.2 | 0.7 ± 0.0 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 |
| Serum Uric acid | mg/dL | 4.2 ± 1.3 | 4.1 ± 0.8 | 4.5 ± 1.3 | 4.8 ± 1.4 | 4.6 ± 1.2 | 4.7 ± 1.2 | 4.3 ± 1.1 | 4.5 ± 1.4 |
| SGOT | IU/mL | 21.4 ± 10.0 | 22.8 ± 13.4 | 25.5 ± 10.3 | 24.6 ± 9.2 | 26.4 ± 18.6 | 22.8 ± 12.1 | 22.3 ± 4.9 | 22.2 ± 4.5 |
| SGPT | IU/mL | 20.8 ± 9.9 | 18.3 ± 10.9 | 23.8 ± 13.2 | 25.0 ± 14.3 | 23.0 ± 8.7 | 23.6 ± 16.1 | 24.6 ± 9.3 | 17.8 ± 5.7 |
| Alkaline Phosphatase | IU/mL | 93.8 ± 40.2 | 149.3 ± 75.7 | 98.4 ± 39.9 | 110.9 ± 42.2 | 86.1 ± 25.1 | 146 ± 94.2 | 72.9 ± 29.1 | 71.7 ± 38.4 |
| GGTP | IU/mL | 28.9± 15.0 | 21.6 ± 12.2 | 23.0 ± 11.2 | 20.5 ± 9.5 | 34.4± 16.4 | 27.0 ± 12.8 | 25.3 ± 9.5 | 22.1 ± 11.9 |
| Total protein | g/dL | 7.2± 0.5 | 7.1 ± 0.4 | 7.0 ± 0.5 | 6.9 ± 0.4 | 7.0± 0.5 | 7.1 ± 0.5 | 6.8 ± 0.3 | 6.8 ± 0.4 |
| Albumins | g/dL | 4.0 ± 0.4 | 3.8 ± 0.2 | 3.8 ± 0.2 | 3.8 ± 0.1 | 3.9 ± 0.4 | 3.8 ± 0.2 | 3.8 ± 0.3 | 3.7 ± 0.2 |
| Globulins | g/dL | 3.1 ± 0.7 | 3.3 ± 0.4 | 3.2 ± 0.3 | 3.0 ± 0.4 | 3.1 ± 0.3 | 3.3 ± 0.4 | 3.0 ± 0.3 | 3.0 ± 0.3 |
| A/G Ratio | ----- | 1.3 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.1 |
| Total Serum bilirubin | mg/dL | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 |
| Serum bilirubin (direct) | mg/dL | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| Serum bilirubin (indirect) | mg/dL | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Total Cholesterol Levels | |||||||||
| Total Cholesterol | mg/dL | 187.5±33.1 | 167.6±29.0 | 174.7 ± 32.0 | 178.1 ± 24.9 | 197.2 ± 55.6 | 189.9 ± 32.9 | 183.8 ± 43.8 | 165.2 ± 22.4 |
| Serum triglycerides | mg/dL | 194.7±71.0 | 140.9 ± 97.6 | 138.9 ± 95.8 | 110.8 ± 54.7 | 164.3± 81.5 | 132.9 ± 61.1 | 162.4 ± 70.1 | 148.0 ± 94.5 |
| S. HDL | mg/dL | 39.5±8.0 | 38.1 ± 8.1 | 40.6 ± 6.6 | 37.6 ± 3.3 | 42.7 ± 7.5 | 38.3 ± 6.4 | 40.6 ± 6.5 | 40.2 ± 13.7 |
| LDL | mg/dL | 111.1±35.8 | 100.6 ± 24.9 | 104.5 ± 30.9 | 117.4 ± 21.4 | 117.8 ± 43.5 | 129.6 ± 31.5 | 110.6 ± 36.4 | 90 ± 35.9 |
| VLDL | mg/dL | 38.9±14.3 | 28.1 ± 19.6 | 29.3 ± 18.2 | 23.8 ± 10.1 | 32.9 ± 16.5 | 32.8 ± 28.3 | 32.6 ± 14.1 | 29.6 ± 19.0 |
| Total cholesterol/ HDL Ratio | 4.8±0.9 | 4.6 ± 1.2 | 4.5 ± 0.7 | 5.0 ± 0.7 | 4.6 ± 1.0 | 5.1 ± 1.0 | 4.7 ± 0.9 | 4.6 ± 0.8 | |
Table 2: Serum Biochemistry test results.
Other than some minor changes none of the biochemical parameters showed any significant variation in the results during the study period. The minor changes observed were not clinically significant. All the data were statistically analyzed and found no significant differences from the normal range in both the study groups. These findings have demonstrated the safety of CPT in humans. Moreover the US Food and Drug Administration (US FDA) has classified gelatin and collagen peptide as a Generally Recognized as Safe (GRAS) product.
Based on the statistical analysis of the data from this clinical study, 5 g of Collagen Peptide is recommended as the ideal add on nutritional therapy for safe for reducing the blood sugar levels significantly and improving the quality of life of the subjects; upon treatment for 12 weeks. Safety parameters were also monitored in the study. The data of the primary parameters studied was used to study the efficacy of the product. The number of subjects of each group showing improvement in visit 5 is tabulated in Table 3.
| Treatment | No. of subjects | FBG | HbA1c | HOMA IR | QoL |
|---|---|---|---|---|---|
| Efficacy points targeted | < 125 mg/dL | 5.7 - 6.4% | > 15% red | >15% score | |
| CPT 2.5 | 20 | 0 | 4 | 0 | 16 |
| CPT 5.0 | 21 | 21 | 20 | 10 | 21 |
| RD 2.5 | 10 | 0 | 0 | 0 | 0 |
| RD 5.0 | 10 | 1 | 0 | 2 | 5 |
Table 3: Efficacy data at V5.
The FBG level and the quality of life based on QoL score was improved in 100% subjects supplemented with 5 g CPT. HbA1c was reduced to the desired level in 95% subjects and the HOMA IR reduction of 48% was attained in the study group. Together these significantly decreased levels of FBG and HbA1c indicate that glucose metabolism improved in 5 g CPT treated subjects. In intergroup comparison, the results show that percentage of improvements is significantly higher in CPT group compared to RD groups. The resistant dextrin used as a control has also been reported to have the following improvement in blood glucose level. A meta-analysis of 37 cross-over Randomised Controlled Trials has revealed the attenuation of postprandial blood glucose in healthy subjects administered with RD [20] Furthermore, the glucose tolerance improving effect [21] in rats and the insulin resistance improving effect [22] in type 2 diabetes patients have been reported. The difference in efficacy levels indicates the difference in mechanism of action between CPT and RD may have role in managing the Type 2 diabetes. The beneficial role of RD is the delayed rate at which the glucose is absorbed from the small intestine [17]. In this study, the improvement in subjects ingested with collagen peptide is significantly better compared to subjects who took oral consumption of RD. Moreover the combination of medicine and CPT did not result in hypoglycemia (<70 mg/dL) in diabetic patients. Thus the efficacy of naturally derived collagen peptide is an advantage as a safe treatment option against type 2 diabetes mellitus. No adverse event was reported by any subject enrolled in the study. There is no selection bias or attrition bias in the study design and hence not mentioned.
Proteins are well known precursors of bioactive peptides showing physiological effects in the body in addition to the nutritional value. Therefore, food derived proteins can be used as potent and safe therapeutic agent in management of diseases [18]. Bioactive peptides have been identified as functional food ingredients in nutraceutical foods providing well-being benefits. It has been demonstrated that some proteins, protein hydrolysates, bioactive peptides and amino acids can control glucose levels directly or indirectly [23]. Current synthetic antidiabetic drugs may result in risks of hypoglycemia, weight gain [24], high background risk of pancreatitis [25] and gastrointestinal side effects [26]. As the development of new therapeutic agents for type-2 diabetes mellitusrelated complications is greatly significant, new approaches are being developed for the management of Type 2 diabetes, like the DPP-4 inhibition treatment pathway [27]. Peptides derived from collagen have been shown to possess DPP-4 inhibitory property, which is an important mechanism in the treatment of Type 2 diabetes. The inhibition of DPP-4 increase the level of incretin hormones and subsequent insulin sensitization and thereby control the blood glucose level in persons with diabetes [28]. A study has been carried out previously to establish the effect of collagen peptides as add on food supplement in management of Type 2 Diabetes [18]. The results of the study had demonstrated that a daily oral dosage of 10 g collagen peptide is effective in controlling the diagnostic parameters of Type 2 Diabetes. The reduced dosage (5 g) of the CPT for the management of Type 2 Diabetes is investigated in the present study and the statistical data confirms the effect of the lower dosage of product in subjects.
Fish scale collagens appear to be an ideal collagen source for the production of peptides as they are rich and sustainable. A few fish protein hydrolysates have shown in vivo glucose uptake-stimulating activity and thereby hyperglycaemia management in addition to regular therapy. These glucose uptake stimulating hydrolysates can ameliorate glucose tolerance either by stimulating glucose uptake via a different mechanism to that of insulin or by increasing insulin sensitivity in target cells [27]. It has been demonstrated that collagen hydrolysates are the richest sources of DPP-4 inhibitory peptides [29]. Gly-Pro-Hyp in the collagen hydrolysates is suggested to be mainly responsible for the DPP-4 inhibition in vitro [30]. The results of the current study have demonstrated that a dosage of 5 g of collagen peptide is effective in lowering the FBG and HbA1c levels and increasing the insulin sensitivity compared to subjects ingested with RD suggesting that the oral ingestion of collagen peptide has resulted in improved glucose metabolism. The product can be further investigated to understand the safety aspects of the product in healthy volunteers and it can also be investigated as a product which can prevent Type 2 diabetes.
To summarize, the present study clearly established the efficacy of the oral dosage of 5 g collagen derived peptides as add on supplement in the management of Type 2 diabetes mellitus. The FBG and HbA1c has reduced to the normal levels at the end of study period in the 5 g CPT group. This demonstrates collagen peptide as one of the emerging treatment option to reduce the dosage of various types of drugs in treatment of Type 2 diabetes and thereby minimizing the deleterious effects of synthetic drugs. Thus based on the statistical analysis of the data from this clinical study, a daily dosage of 5 g Collagen Peptide is recommended as the ideal add on nutritional therapy for safe for the management of Type 2 diabetes reducing the blood sugar levels significantly and improving the quality of life of the subjects.
This publication is dedicated to Dr. J S Suresh Kumar, who made great contribution in designing and executing this clinical study (demised on 27/08/2018). We pray for his soul.
Citation: Devasia S, Kumar S, Stephena PS, Inoue N, Sugihara F, Koizumi S, et al. (2020) A Double Blind, Randomised, Four Arm Clinical Study to Evaluate the Safety, Efficacy and Tolerability of Collagen Peptide as a Nutraceutical Therapy in the Management of Type II Diabetes Mellitus. J Diabetes Metab 10:839. doi: 10.35248/2155-6156.19.10.839.
Received: 10-Sep-2019 Published: 20-Jan-2020, DOI: 10.35248/2155-6156.20.11.839
Copyright: © 2020 Devasia S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.