Research Article - (2021) Volume 12, Issue 10
Objectives: Metabolic syndrome is a cluster of metabolic disorders including obesity, hypertension, diabetes mellitus and dyslipidemia. Insulin resistance is the underlying pathological condition for the development of metabolic syndrome. Microalbuminuria is also associated with some conditions like obesity, hypertension and diabetic nephropathy. Dyslipidemia is commonly found in obesity, diabetes mellitus and is frequently measured in our day to day clinical settings. Besides this Tg-HDL ratio is used for risk assessment coronary artery disease now a days. Therefore any association of Tg-HDL ratio with metabolic syndrome and Microalbuminuria can help our clinicians to predict metabolic derangements earlier and easily.
Study design: In this cross sectional analytical study 150 subjects were included by convenient sampling from outpatient department of endocrinology of a tertiary level hospital, Dhaka, Bangladesh.
Methods: A quantitative research has been performed to observe the relationship between the components of metabolic syndrome patients. Different statistical analysis including binary logistic regression has been performed to see the association between them.
Results: In this study, male–female ratio was almost equal, with average age 42.72(7.94).± Among the study subjects 55 were non-MetS and rest of the candidates were with MetS. Among the study subjects for presence of 3, 4 & 5 components of MetS, the average Tg-Hdl ratios were found to be 5.01, 5.76, 6.17 and risk of having higher Tg-Hdl values were 1.92, 2.26 & 2.39 times higher respectively than those without MetS. Again Risk of MetS increased by 0.53, 1.17 & 1.56 times in Q2, Q3 & Q4 respectively in comparison to Q1 of the Tg-HDL ratio of the participants. Binary logistic regression analysis was done to find out relationship between MetS and microalbuminuria with Tg-HDL ratio and was found significant. Odds ratios for MetS and microalbuminuria with respect to Tg-HDL ratio were observed as 1.30 and 1.19 with β coefficients 0.267 and 0.177 respectively. The result implies 30% and 19% more risk of developing Mets and microalbuminuria for patients with high Tg-HDL ratio (p<0.05 for all cases).
Conclusion: Tg-HDL ratio level strongly correlated with both MetS and microalbuminuria. Risk of an individual to develop MetS and microalbuminuria for high Tg-HDL ratio was found significantly higher than normal individuals. So early treatment for high Tg-HDL ratio can effectively decline the rate of progression of MetS as well as can diminish the risk of development of microalbuminuria.
Metabolic syndrome (MetS); Microalbuminuria; Triacyl glycerol-high density lipoprotein cholesterol ratio (Tg-HDL); Albumin-creatinine ratio (ACR)
BMI- Body Mass Index; SBP- Systolic Blood Pressure; DBP-Diastolic Blood Pressure; WC- Waist Circumference; FBGFasting Blood Glucose; T.Chol- Total Cholesterol; TG- Tri Acyl Glycerol; LDL-c- Low Density Lipoprotein Cholesterol; HDL-c-High Density Lipoprotein Cholesterol; UAC – Urinary Albumin Concentration; UCR- Urinary Creatinine Excretion Rate; ACR – Urinary Albumin-Creatinine Ratio; Tg-HDL- Triglyceride-HDL ratio; T.Chol-HDL- Total Cholesterol- HDL ratio.
Metabolic syndrome is a common public health problem in our country and its incidence gradually increasing both in urban and rural area day by day. It consists of central obesity, hypertention, insulin resistance (IR) and dyslipidemia [1]. Insulin resistance is the major underlying pathological condition for the development of metabolic syndrome (MetS), atherosclerosis and diabetes mellitus. Besides these microalbuminuria was included in diagnostic criteria of MetS by the World Health Organization (WHO) in 1998 [2]. Again, it is an early warning sign for diabetic nephropathy and CKD [3]. Additionally it has been known as a useful predictor of cardiovascular events among adults in some studies abroad [4].
Therefore it is crucial to measure insulin resistance (IR) among subjects with MetS and microalbuminuria. The homeostasis model assessment of IR (HOMA-IR) is a marker of IR where fasting insulin level have to be measured to calculate HOMA-IR. Measurement of fasting insulin level is not routinely asked in day to day clinical practice. Several studies carried out in abroad showed strong correlation of triacyl glycerol- high density lipoprotein cholesterol ratio (Tg-HDL) with IR [5]. Again Tg-Hdl ratio has been shown to be better screening tool for MetS than HOMA-IR in some studies [6, 7]. The gold standard for measuring IR is hyperinsulinemic-euglycemic clamp [8] and most popular alternative is HOMA-IR [9]. However their high cost and poor accessibility and reproducibility confined them only in research lab [8]. Alternative easily accessible markers are tried to develop for the assessment of IR in clinical practice [10]. Tg-HDL ration showed the promise in studies conducted abroad [6, 7, 11].
However there is very limited number of studies conducted in our population to investigate any relationship between Tg-HDL ratio with metabolic syndrome and micro albuminuria. Therefore this study was designed to evaluate the relationship between Tg-HDL ratios with metabolic syndrome along with microalbuminuria in our population.
Study design
This cross-sectional analytical study was done in the department of Bio-chemistry of Sir Salimullah Medical College, Dhaka, Bangladesh. We have collected our data from the study participants (N = 150) who had fulfilled the enrollment criteria attending the outpatient department of Endocrinology of Mitford Hospital, Dhaka from December, 2017 to November, 2018. We have included subjects of both sex aged between 19 to 59 years and free from any chronic diseases like stroke, chronic kidney disease, chronic liver disease, congestive cardiac failure, lymphoma and cancer. We also excluded pregnant women and lactating mother and those having self-reported hepatitis C, hepatitis B and HIV infection or on drugs like angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for lowering blood pressure and having history of alcohol consumption or cigarette smoking. The study was approved by the institutional Ethical committee of Sir Salimullah Medical College, Dhaka, Bangladesh.
The approval number is ref:SSMC/Dhaka/2017/29 and the date of approval is 21.08.2017. All study subjects were informed about the aim of the study and informed written consent was taken before personal data and sample was taken.
General data collection
Personal information, anthropometric data, lifestyle factors was collected in a separate questionnaire. Body weight was measured to the nearest of 0.10 kg by digital weighing machine, height, waist circumference was measured by measuring tape following WHO protocol to the nearest of 0.10 cm [12]. Blood pressure was measured by sphygmomanometer (LP K2, Japan) in standard sitting condition and average value of the two consecutive measurements taken five minutes apart was used for calculation.
Sample collection and laboratory analysis
The study subjects were advised to fast overnight and neither to do any exercise nor to drink excess water prior to give urine and venous blood. Five ml of venous blood was taken and serum was separated following thirty minutes standing in room temperature and centrifuging at 3000 rpm for 10 minutes.
Fasting blood glucose was measured on the day of sample collection and lipid profile was measured weekly by batch analysis in semi auto analyzer after being preserved at -20ºc. Five ml spot urine sample was collected in separate test tube and was used for measuring urinary creatinine concentration as well as urinary albumin concentration by Jaffe’s kinetic alkaline picrate method and immunoturbidimetric method respectively. Then urinary albumin: creatinine ratio was calculated for each preserved sample of respective individual. The standard calibration measures were taken to achieve accurate data. Rest of the collected samples was preserved at -70ºc for future analysis.
Diagnostic criteria for metabolic syndrome (MetS)
MetS was diagnosed on the basis of criteria proposed by National Cholesterol Education Program- Adult Treatment Pannel-III (NCEP-ATP-III) [13]. MetS was defined if three or more of the following five defining characteristics present: i) Elevated blood pressure (systolic blood pressure (SBP) ≥130 mmHg and/ or diastolic blood pressure (DBP) ≥ 85 mmHg) or on drug therapy for high blood pressure; ii) raised waist circumference (WC) specific for race ethnicity (≥ 102 cm for men and ≥ 88 cm for women); iii) hypertriglyceridemia (triacyl glycerol (Tg) > 150 mg/dl) or on drug therapy for elevated Tg; iv) hyper glycemia (fasting blood glucose (FBG) ≥ 100 mg/dl) or on drug therapy for elevated blood glucose; v) low high density lipoprotein cholesterol (HDL-c) (< 50 mg/dl for women and < 40 mg/dl for men) or on drug therapy for low HDL-c. Microalbuminuria was defined if urinary albumin to creatinine ratio (ACR) value lies between ≥ 30 to 300 mg/g. ACR value < 30 mg/g was denoted as normoalbuminuria [14].
Statistical analysis
All data were analyzed by statistical package for social science (SPSS) version 23. Data was presented as mean ± standard deviation (SD). Categorical variables were analyzed using Chi-square test. Comparison between groups was done by independent t-test. Relationship between Tg-HDL ratio with MetS and microalbuminuria were determined by individual binary logistic regression analysis. Risk association was determined by measuring odds ratio (OR) and confidence interval (CI). P-values < 0.05 were considered statistically significant.
One hundred and fifty adult subjects ranging from 19 to 59 years was included in this study with mean age 42.72 (±7.94). Among them 76 were male and 74 were female. Base line characteristics of the study subjects were shown in Table 1.
| Variables | Mean | ± Standard Deviation (SD) |
|---|---|---|
| Age | 42.72 | 7.94 |
| BMI | 27.21 | 3.36 |
| SBP | 137.11 | 22.06 |
| DBP | 88.65 | 9.97 |
| WC | 93.89 | 7.03 |
| FBG | 9.412 | 3.35 |
| TC | 216.20 | 42.07 |
| Tg | 199.10 | 81.86 |
| Ldl-c | 139.80 | 39.53 |
| Hdl-c | 37.41 | 5.81 |
| UAC | 50.93 | 56.99 |
| UCR | 187.98 | 94.81 |
| ACR | 29.48 | 39.01 |
| Tg-HDL | 5.48 | 2.44 |
| TC-HDL | 5.91 | 1.41 |
Table 1: Baseline characteristics of the study subjects (N=150).
Statistical difference of the variables between subjects with MetS and without MetS was shown in Table 2. Out of one-fifty study subjects 63.33% were suffering from MetS and rest were non-MetS. Among them subjects with MetS were older than those from non- MetS 43.55(±7.99) vs 41.29(±7.72). Between these two groups, subjects with MetS have significantly higher average WC, SBP, DBP, FBG, ACR and significantly lower HDL-c. Difference of Tg- HDL ratio & TC- HDL ratio are also statistically significant among subjects in MetS group than those in non-MetS group.
| Variable | MetS(n=150) | Microalbuminuria (n=150) | ||||
|---|---|---|---|---|---|---|
| Metabolic Syndrome (n=95) | Non-Metabolic Syndrome (n=55) | p value | Normoalbuminuric (n=98) | Microalbuminuric (n=52) | p value | |
| Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | |||
| Age | 43.55(±7.99) | 41.29(±7.72) | 0.094 | 41.80(±7.68) | 44.46(±8.20) | 0.05* |
| BMI | 27.18(±3.44) | 27.23(±3.23) | 0.932 | 26.86(±3.28) | 27.86(±3.43) | 0.081 |
| WC | 95.22(±7.02) | 91.60(±6.49) | 0.002* | 93.26(±6.89) | 95.10(±7.20) | 0.13 |
| SBP | 142.22(±20.45) | 128.29(±22.10) | 0.000* | 130.38(±18.10) | 149.81(±23.39) | 0.00* |
| DBP | 90.17(±8.73) | 86.02(±11.42) | 0.013* | 85.98(±8.31) | 93.67(±10.93) | 0.00* |
| FBG | 9.79(±3.41) | 8.74(±3.15) | 0.006* | 8.83(±3.19) | 10.51(±3.40) | 0.00* |
| Tg | 203.95(±73.98) | 190.73(±94.07) | 0.342 | 185.34(±52.91) | 225.04(±114.92) | 0.00* |
| HDL-c | 35.35(±4.42) | 40.96(±6.22) | 0.000* | 37.32(±5.61) | 37.58(±6.21) | 0.79 |
| TC | 213.12(±40.28) | 221.53(±44.86) | 0.239 | 215.60(±41.78) | 217.33(±42.98) | 0.82 |
| Tg-HDL | 5.91(±2.35) | 4.74(±2.44) | 0.004* | 5.11(±1.83) | 6.17(±3.22) | 0.01* |
| TC- HDL | 6.12(±1.38) | 5.53(±1.39) | 0.013* | 5.92(±1.50) | 5.88(±1.26) | 0.88 |
| ACR | 31.72(+-42.19) | 25.6(±32.81) | 0.357 | 10.37(±5.98) | 65.48( ±48.53) | 0.00* |
Table 2: Variables according to status of metabolic syndrome and microalbuminuria.
Baseline characteristics of the study subjects among the two groups on the basis of their albuminuric status were also shown in table 2.
Among the study subjects 52 (34.66%) were microalbuminuric with mean age 44.46 (±8.20) and rest are normoalbuminuric 98(65.34%) with mean age 41.80(±7.68). Among the five defining components of MetS high SBP, DBP, FBG & TG is significantly higher among subjects with microalbuminuria. Tg-HDL ratio shows statistically significant higher values in groups with microalbuminuria than those with normoalbuminuria. Whereas the difference of TC-HDL ratio between normoalbuminuric and microalbuminuric subjects was insignificant.
Range of Tg-Hdl ratio of the study subjects were (2.19-17.9) divided into four quartiles. Means (±SD) of the components of MetS including other related variables like age, height, weight, BMI, TC and ACR values were demonstrated among the quartiles of Tg- HDL ratio of the study population in Table 3. A binary logistic regression model (Table 4) has been set to show the significance of Tg-HDL ratio in relation with MetS as a whole. Here Tg-HDL ratio shows significant association (Chi square =9.589 with df=1, p value=0.002) among subjects with MetS. From this study, it is also observed that for each unit increase in Tg-HDL ratio, patients grow 30% more risk of metabolic syndrome.
| variable | Q1 (2.19-3.95) | Q2 (3.95-4.67) | Q3 (4.67-6.26) | Q4 (6.26-17.97) |
|---|---|---|---|---|
| Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | |
| Age | 43.18 (8.05) | 42.95 (8.98) | 41.95 (6.79) | 42.79 (8.08) |
| Height | 1.54 (0.08) | 1.54 (0.07) | 1.55 (0.08) | 1.56 (0.08) |
| Weight | 64.79 (9.15) | 63.65 (8.43) | 66.32 (13.39) | 65.87 (9.66) |
| BMI | 27.43 (3.06) | 26.78 (2.69) | 27.65 (4.26) | 26.97 (3.3012) |
| SBP | 140.42 (21.89) | 134.08 (19.63) | 134.05 (21.11) | 139.74 (25.20) |
| DBP | 91.05 (10.34) | 86.68 (8.85) | 87.43 (10.38) | 89.34 (10.01) |
| WC | 94.63 (6.39) | 92.97 (6.77) | 94.27 (7.77) | 93.68 (7.32) |
| FBG | 8.50 (3.067) | 8.81 (3.064) | 10.33 (3.88) | 10.01 (3.10) |
| Tg | 138.66 (24.36) | 169.78 (19.17) | 191.73 (22.53) | 295.26 (106.68) |
| HDL-c | 41.16 (6.54) | 39.27 (4.51) | 35.89 (4.73) | 33.32 (3.76) |
| TC | 201.42 (38.08) | 220.22 (44.18) | 216.57 (37.57) | 226.71 (45.27) |
| ACR | 24.32 (25.817) | 22.43 (25.478) | 39.57 (58.25) | 31.66 (36.67) |
Table 3: Characteristics according to quartile (n=150).
| Variable | Coefficient (β) | P value | OR (exp β) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| Tg-HDL ratio | .267 | .007* | 1.306 | 1.077 | 1.584 |
| Constant | -.849 | .103 | .428 |
Table 4: Binary logistic regression of Mets with respect to Tg-HDL ratio.
Risk of metabolic syndrome increases in higher quartiles of Tg- HDL ratio has shown in Table 5. Risk of MetS increased by 0.53, 1.17 and 1.56 times in Q2, Q3 and Q4 respectively in comparison to Q1.
| Q2 | Q3 | Q4 | |
|---|---|---|---|
| OR | 0.53 | 1.17 | 1.56 |
| 95% CI | (0.19,1.49) | (0.46, 3.01) | (0.62,3.93) |
Table 5: Incremental OR with 95% CI for quartiles against Tg-HDL (cutoff values are 3.948, 4.671, 6.259).
In this following Figure 1 we have focused the rising trend of Tg- HDL ratio among subjects having increasing number of MetS components. Mean of Tg-HDL ratio for subjects having ≤ 2 components of MetS was 3.93. whereas with presence of higher number of components among subjects with MetS having mean Tg-HDL ratio 5.01, 5.76, 6.17 respectively for presence of 3, 4 and 5 copmonents of MetS.
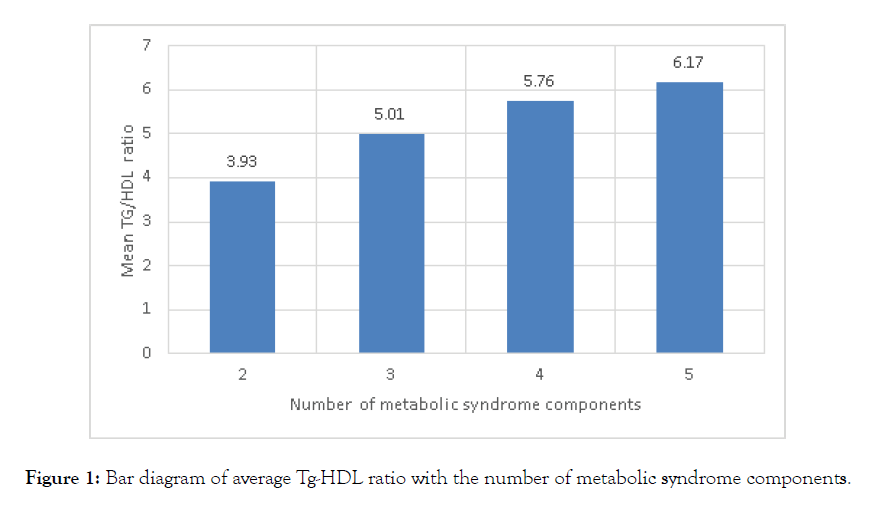
Figure 1: Bar diagram of average Tg-HDL ratio with the number of metabolic syndrome components.
Severity of metabolic syndrome as indicated by presence of increasing number of its components is associated with higher values of Tg-HDL ratio has been demonstrated in table 6. Risk of having higher values of Tg-HDL ratio for presence of 3, 4 & 5 defining components of MetS were 1.92, 2.25 & 2.39 times than subjects without MetS.
| 3 | 4 | 5 | |
|---|---|---|---|
| OR | 1.919 | 2.255 | 2.386 |
| 95% CI | (1.054,3.495) | (1.245, 4.083) | (1.310,4.344)) |
Table 6: Incremental OR for number of MetS components against Tg-HDL ratio.
Binary logistic regression model was set to show the risk of microalbuminuria in respect to TC-HDL ratio and Tg-HDL ratio in table 7 respectively. From statistical point of view we can make an inference that microalbuminuria is significantly associated with Tg-HDL ratio whereas TC-HDL ratio is not associated with microalbuminuria. From this study, it is observed that there is a 19% increase in risk of microalbuminuria for each unit increase in Tg-HDL ratio of patients.
| Variable | Coefficient (β) | P value | OR (exp β) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| Tg-HDL ratio Constant |
.177 -1.619 |
.017* .000 |
1.193 .198 |
1.032 | 1.380 |
| TC-HDL ratio Constant |
-.019 -.522 |
.877 .481 |
.981 .593 |
.772 | 1.246 |
Table 7: Individual Binary logistic regression of microalbuminuria with respect to Tg-Hdl ratio and TC-HDL ratio.
Incidence of MetS is increasing in our population day by day. Insulin resistance is the major pathological condition behind it. Dyslipidemia is an integral part of MetS and also frequently found in diabetes mellitus, obesity and hypertension. Besides this dyslipidemia is used as a predictor for coronary artery disease. Microalbuminuria was also used as diagnostic criteria for MetS by WHO [2]. It is also an early marker for diabetic nephropathy [3]. So this study have been designed to find out any correlation between Tg-Hdl ratio with MetS as well as microalbuminuria in our population. In this study a total of one hundred and fifty subjects were included by convenient sampling. Among the study subjects (150) male were 76(50.66%) female were 74(49.33%) and their mean age was 42.72(±7.94). Among them 95(63%) were with MetS and rest are non-MetS. Among them subjects with MetS were older in average than those without MetS (43.55(±7.99) vs 41.29(±7.72)). Between these two groups subjects with MetS have significantly higher WC, SBP, DBP, FBG, ACR and significantly lower HDL-c. Difference of Tg-HDL ratio & TC-HDL ratio were also statistically significant among subjects in MetS group than those off non-MetS group. A similar type of study conducted by Shin HG et al. [15] among Korean adults found statistically significant difference for age, high WC, BP, FBG and low HDL-c among subjects with MetS in comparison to non-MetS group. They also found statistically significant difference for Tg-HDL ratio between the groups created on the basis of presence of MetS defining criteria. They did not showed any relationship with the status of microalbuminuria and TC-HDL ratio.
In this study we showed among the study subjects 52(34.66%) were microalbuminuric with mean age 44.46(± 8.20) and rest are normoalbuminuric 98(65.34%) with mean age 41.80(pm7.68). Among the five defining components of MetS high SBP, DBP, FBG & TG were significantly higher among subjects with microalbuminuria. Tg-HDL ratio shows statistically significant high value among subjects with microalbuminuria than those with normoalbuminuria. Whereas difference of TC- HDL ratio between normoalbuminuric with microalbuminuric subjects were insignificant. In a population based study conducted by Sheng CS et al. [16] in China showed significant relationship of microalbuminuria with high BP and FBG among five defining components of MetS. Another study among Japanese adults by Hao et al. [17] found significant association of microalbuminuria with elevated WC, BP, FBG among five defining criteria for MetS. Whereas a large population based study conducted over two years by Lee et al. [18] in Korea found significant association between microalbuminuria with MetS and each of its components in both sex after adjusting data for covariates.
In our study we have focused the rising trend of Tg-HDL ratio among subjects having increasing number of MetS components. Mean of Tg-HDL ratio for subjects having 2 components of MetS was 3.93. Whereas among subjects with MetS having mean Tg- HDL ratio 5.01, 5.76, 6.17 respectively for presence of 3, 4 & 5 copmonents of MetS. In a study carried out by Sheng CS et al. [16] showed Tg-HDL ratio for having 2,3,4 & 5 components of MetS were 4.20, 6.17, 7.33 & 10.92 respectively in men and a similar rising trend also observed among women. In a study carried out by Moriyama K. [5] among healthy Japanese showed similar increasing trend of Tg-HDL ratio along with presence of increasing number of defining components of MetS among both sex. Risk of having higher values of Tg-HDL ratio for presence of 3, 4 & 5 defining components of MetS were 1.92, 2.25 & 2.39 times than subjects without MetS in our study. Again Risk of MetS increased by 0.53, 1.17 & 1.56 times in Q2, Q3 & Q4 respectively in comparison to Q1 of Tg-HDL ratio after dividing the range of Tg-Hdl ratio of this study subjects (2.19-17.9) into four quartiles. This analysis reflects how strongly high Tg-HDL ratio can cause MetS and severity of MetS has high chance of having high Tg and low HDL-c values. In other words we may conclude that probability of having MetS in a subject is high when there is high Tg-HDL ratio. So this finding can help our clinician to look for the development of MetS earlier than usual in their day to day clinical practice. Again this finding was also supported by making another statistical analysis when a binary logistic regression model was set to show the significance of Tg-HDL ratio in relation with MetS as a whole. Here Tg-HDL ratio shows significant correlation among subjects with MetS. This finding was supported by a study conducted in Korean adolescent age group ranging from 10 to 18 years conducted by Chu SY at al. [19] from 2008 to 2010, as they showed higher risk association of both TC-HDL ratio and Tg-HDL ratio above the cut off values were significantly high. Another study conducted among Korean adults aged ≥20 years by Shin et al. [15] found risk of MetS is high in upper quartile Tg-HDL ratio and also mean of Tg-HDL ratio is high with presence of increased number of MetS components. This finding can be explained by insulin resistance which is associated with obesity can facilitate progression to diabetes mellitus and also hypertension as induces endothelial dysfunction of blood vessels due to oxidant stresses causing inflammation and ultimately atherosclerosis. This cascade of vascular endothelial damage ultimately leads to microalbuminuria and diabetic nephropathy [20–22].
In our study we have also found that for a unit change in Tg- HDL ratio there is 19% increased risk for the development of microalbuminuria in patients. This finding supports the pathophysiology of vascular endothelial damage caused by high Tg- HDL ratio.
In this study we have found Tg-Hdl ratio is strongly associated with MetS and microalbuminura. So this ratio can be used to predict the risk of development of both of these events earlier in our clinical practice. It’s use can become an affordable, available and alternative tool for both of these clinical conditions which demand early intervention and follow-up.
This study was conducted in a small population and in a single center which is unable to reflect the actual condition of our general population. So it demands large scale, multicenter, population based study and cut off value for Tg-HDL ratio for our population should be found out.
The authors wish to thank healthcare technicians for their assistance and show gratitude to all the participants of this study for their willing and active participation.
MMS played the major role in sample collection and analysis. MMS and RH played a role in conception making and designing the study. MMS and FAT take part in data collection. ST along with MMS took part in data analysis and interpretation and together wrote the manuscript. All authors go through the manuscript and approved the final version.
Citation: Saadi MM, Haque R, Tania FA, Tasnim S (2021) Association of Tg-HDL Ratio with Metabolic Syndrome and Its Components Including Microalbuminuria. J Diabetes Metab. 12:899. doi: 10.35248/2252-5211.21.12.899
Received: 11-Sep-2021 Published: 27-Oct-2021, DOI: 10.35248/2155-6156.21.12.899
Copyright: © 2021 Saadi MM, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.