Research Article - (2021) Volume 12, Issue 8
Background/Aims: Nonalcoholic fatty liver disease (NAFLD) is believed to be the most common chronic liver disease. The present study aimed to evaluate the efficacy of the vildagliptin versus vildagliptin / metformin combination in the treatment of nonalcoholic fatty liver disease patients with type 2 diabetes mellitus (T 2DM).
Methods: One hundred twenty patients, who were newly diagnosed with T 2DM, and proof of diagnosis of NAFLD, were enrolled in a twelve-month open label prospective parallel study. The patients were divided into two groups; Group 1 received vildagliptin in a dose of 50 mg twice daily and Group 2 received vildagliptin/metformin in a dose of 50 mg/ 1000 mg twice daily. Body mass index and fatty liver ultrasound grading were evaluated with the assay of HbA1c and serum levels of fasting and postprandial glucose, lipid profile, liver enzymes, fasting insulin, adiponectin, ferritin, and creatinine and blood urea with calculation of HOMA-IR before and after treatment.
Results: There was a significant improvement in fatty liver ultrasound grading in both groups (p=0.001). Univariate and multivariate analysis showed that the improvement in NAFLD grading in both groups was affected by change in BMI, HbA1c, fasting insulin, HOMA-IR, triglycerides and adiponectin. After twelve months both groups showed significant decrease in BMI (p=0.001), fasting serum insulin (p<0.05 and p=0.001, respectively), HOMA-IR, HbA1c and triglycerides (p=0.001). However, adiponectin level increased significantly in both groups (p=0.001).
Conclusion: Both vildagliptin or vildagliptin/metformin combination can improve fatty liver disease through their effect on BMI, serum levels of insulin, triglycerides, adiponectin and HOMA-IR.
Nonalcoholic fatty liver; Vildagliptin; Metformin; Type 2 diabetes; Dipeptidyl Peptidase IV (DPP-4) inhibitor
Nonalcoholic fatty liver disease (NAFLD) is believed to be the most common chronic liver disease, affecting at least one-third of the population worldwide [1]. The more aggressive form is known as nonalcoholic steatohepatitis (NASH) which is characterized by hepatocyte necrosis and inflammation [2]. Those patients with NASH are at high risk of cirrhosis and premature mortality, as well as at increased risk of hepatocellular carcinoma [3].
The prevalence of NAFLD is high in patients with T2DM, reflecting the frequent occurrence of obesity and insulin resistance in T2DM [4]. Several lines of evidence have indicated a pathogenic role of insulin resistance, and a strong association between NASH, T2DM and metabolic syndrome [5]. Importantly, NAFLD appears to enhance the risk for T2DM, as well as worsen glycemic control and cardiovascular disease in diabetic patients [6]. The opportunity to take into account NAFLD in T2DM prevention and care has stimulated several clinical studies in which antidiabetic drugs as biguanides and thiazolidinediones were used as therapeutic approaches for NAFLD in recent years [7, 8].
Metformin improves insulin sensitivity and serum alanine transaminase (ALT) and aspartate transaminase (AST) levels in the majority of subjects; however, it has no significant effect on liver histology. Thiazolidinediones improves insulin sensitivity, serum ALT&AST levels and histology in some cases, but there are some concerns about the safety of long-term therapy [7, 8].
Dipeptidyl Peptidase IV (DPP-4) inhibitors were studied to evaluate their effect beyond glucose control. One of the studied effects of some drugs in the DPP-4 inhibitors group was their role in the management of the fatty liver disease. However, few studies are there to address this effect. The findings of the studies were encouraging regarding the improvement of liver enzymes and histopathological response with some contradictory data [9,10].
Vildagliptin is a commonly used DDP-4 inhibitor to treat T2DM. In addition to blood sugar control, vildagliptin has a potential role in the treatment of NAFLD. The proposed mechanism by which vildagliptin produces its beneficial effect in patients of NAFLD includes improvement in insulin resistance, decrease in serum DPP-4 activity which is high in patients with NAFLD and correlates to inflammation, oxidative stress and hepatic steatosis in patients with NAFLD. The beneficial effect of vildagliptin on risk factors which are associated with NAFLD in various clinical as well as in animal studies, such as improvement in metabolic syndrome, blood pressure, weight gain and lipid profiles [11].
This study was conducted to establish the beneficial effect of vildagliptin on the fatty liver disease on a large number of patients for a longer duration in relation to previous studies on DDP-4 inhibitors.
Patients
From February 2017 to December 2020, 120 patients who were newly diagnosed with T2D and proof of diagnosis of NAFLD were selected from Outpatient Clinic of the Endocrine and Diabetes Unit of Tanta University Hospitals, Tanta, Egypt, were included in this study. Inclusion criteria were established diagnosis of NAFLD (based on liver ultrasonography) and T2DM (based on ADA criteria of diagnosis of diabetes), both sex aged ≥ 18 years old.
The exclusion criteria were, patients with known history of viral hepatitis, hemochromatosis, Wilson’s disease, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, biliary obstruction, alpha-1 antitrypsin deficiency, ischemic cardiac or cerebrovascular disease, or malignancies and alcohol intake exceeding 20 g/day. Those patients with history of pancreatitis, or having estimated glomerular filtration rate (eGFR) <60ml/ min/1.73m2 or Child-Pugh score >6 and pregnant female patients were excluded from the study. In addition, patients who were taking any angiotensin II receptor blockers, estrogens, amiodarone, corticosteroids, tamoxifen, lipid lowering agents, and other antidiabetic medications were also ruled out.
Patients were divided into two groups: group 1 included 60 patients with HBA1c < 7.5% on diagnosis, and received vildagliptin in a dose of 50 mg twice daily and group 2 included 60 patients had HBA1c (7.5%-<9%) on diagnosis and received vildagliptin/ Metformin in a dose of 50 mg/1000 mg twice daily.
Methods
All patients were subjected to full history taking and clinical examination. Also, they underwent liver ultrasound study and laboratory tests at baseline and twelve-month post treatment.
All blood samples were obtained after a 10- to 12-hours fasting period. Then serum was then separated after centrifugation coded and stored at -80°C until analysis. The rest portion of blood was collected in tubes containing EDTA and centrifuged immediately for HbA1c measurement.
Anthropometric measures
BMI was calculated as weight in kilograms divided by the square of the height in meters (BMI = weight (kg) / height2 (m)) before and after treatment period [12].
Biochemical assays
The serum levels of fasting (FSG) and post prandial glucose (PPSG), ALT, AST, creatinine, lipid profile and blood urea were measured by automated system (KONILAB model: prime 30 Thermo scientific, USA).
Glycated hemoglobin (HbA1c %): was measured by automated System (H.P.L.C model: G89051, Tosoh, USA).
While adiponectin, ferritin and fasting serum insulin levels were determined using ELISA technique with commercial kits of adiponectin (Sunred Biological Technology Company, china), ferritin (Alkor Bio Company, Russia) and insulin (Immunospec, USA), respectively.
Insulin resistance was calculated using homeostatic model assessment of insulin resistance (HOMA-IR=Fasting insulin concentration (μIU/ml) ×fasting glucose concentration (mg/ dl)/405) [13].
Liver ultrasound
Liver ultrasound was performed at baseline and 12-month posttreatment and evaluated by specialized radiologist. The classification of NAFLD was based upon the severity of fatty liver on abdominal ultrasound according to the given criteria [11].
Grade 0: No fatty liver
Grade 1 (Mild): There was slight diffuse increase in the echogenicity of liver parenchyma or increased hepatorenal contrast with normal diaphragm and intrahepatic vessel borders.
Grade 2 (Moderate): There was moderate diffuse increase in the echogenicity of liver parenchyma and increased hepatorenal contrast with slight impairment of diaphragm and intrahepatic vessel borders.
Grade 3 (Severe): In addition to criteria for moderate steatosis there was no visualization of posterior portion of the right lobe of liver, intrahepatic vessel, borders and diaphragm.
Statistical analysis
All data were expressed as mean ± SD. The mean values within the same group before and after treatment was compared using paired student t-test. The mean values between the two groups before and after the assigned twelve months treatment were compared using unpaired student t-test with statistical significance being inferred at p <0.05. Categorical variables were compared between groups using the chi-squared test. Correlation analysis was done using Spearman correlation. Univariate and multivariate analysis was done on measured variables. Statistical analysis was carried out using SPSS statistical package version 22.0, (August 2013), IBM corporation software group, USA.
Demographic characteristics: Concrete flow diagram for the selection of study subjects was presented in Figure 1. Demographic data of the participants were presented in Table 1. There were no significant differences between both groups in baseline demographics and clinical data except HbA1c was significantly higher in group2 than group 1 (Table 1).
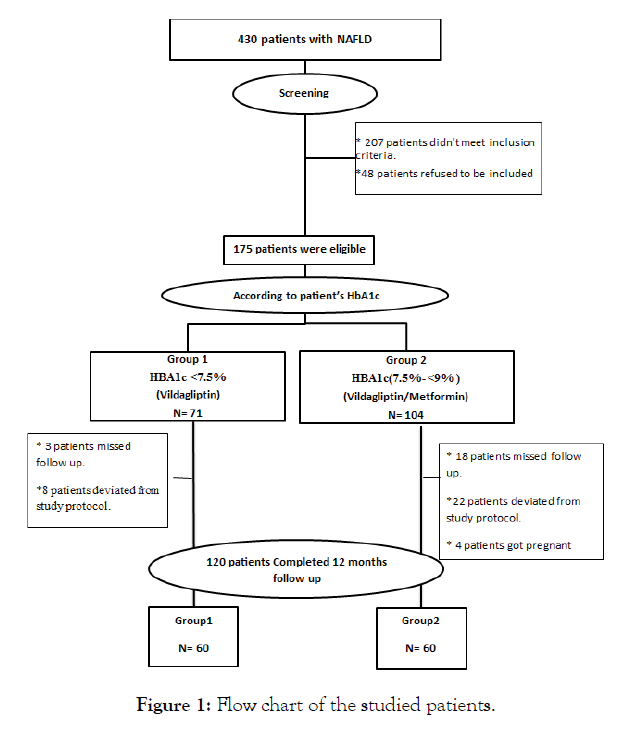
Figure 1: Flow chart of the studied patients.
| Baseline characteristics | Group1 | Group2 | P value |
|---|---|---|---|
| Age (years) | 44.33 ± 9.16(27–61) | 44.25 ± 8.92(28-62) | 0.960 |
| Sex (M/F) | 29/31 | 27/33 | 0.714 |
| Body weight (Kg) | 82.56 ± 10.38 | 82.85 ± 10.31 | 0.878 |
| Height (cm) | 161.73 ± 6.43 | 161.07 ± 6.18 | 0.564 |
| BMI (Kg/m2) | 31.50 ± 2.87 | 31.95 ± 3.66 | 0.461 |
| FSG (mg/dl) | 160.82 ± 34.00 | 161.93 ± 29.82 | 0.849 |
| PPSG (mg/dl) | 318.27 ± 98.13 | 323.38 ± 102.32 | 0.780 |
| BUN (mg/dl) | 29.07 ± 5.56 | 29.33 ± 5.35 | 0.789 |
| S.Cr. (mg/dl) | 0.63 ± 0.20 | 0.61 ± 0.24 | 0.653 |
| HbA1c ( %) | 7.02 ± 0.25 | 8.05 ± 0.46 | 0.001* |
| ALT (IU/L) | 38.58 ± 11.26 | 39.95 ± 11.05 | 0.504 |
| AST (IU/L) | 30.82 ± 6.67 | 31.69 ± 5.51 | 0.435 |
| TC (mg/dl) | 183.73 ± 17.85 | 182.60 ± 17.41 | 0.725 |
| TG (mg/dl) | 146.63 ± 18.56 | 146.25 ± 20.46 | 0.915 |
| LDL-C (mg/dl) | 111.44 ± 24.59 | 113.73 ± 26.03 | 0.621 |
| HDL-C (mg/dl) | 42.97 ± 17.01 | 40.30 ± 18.30 | 0.118 |
| Fasting insulin (µIU/ml) | 13.30 ± 4.89 | 13.22 ± 5.08 | 0.936 |
| HOMA-IR | 5.29 ± 2.23 | 5.25 ± 2.09 | 0.924 |
| Adiponectin (µg/ml) | 7.15 ± 2.35 | 6.79 ± 2.50 | 0.678 |
| Ferritin (ng/ml) | 178.71 ± 84.77 | 188.63 ± 83.19 | 0.519 |
| SBP (mmhg) | 124.55 ± 9.68 | 125.23 ± 10.32 | 0.709 |
| DBP (mmhg) | 77.05 ± 6.25 | 78.28 ± 6.73 | 0.301 |
| AHDs % | |||
| Calcium channel blocker | 6.7 | 8.3 | 0.729 |
| Beta blocker | 3.3 | 5 | 0.648 |
Data are presented as mean ± SD. M: male; F: female; BMI: body mass index; FSG: fasting serum glucose; PPPSG: post prandial serum glucose; BUN: blood urea nitrogen; S.Cr.: serum creatinine; HbA1c hemoglobin A1c (glycated hemoglobin); AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TC: Total cholesterol; TG: Triglycerides; LDL-C: Low density lipoprotein; HDL-C: High density lipoprotein; HOMA-IR: Homeostatic model assessment of insulin resistance; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; AHDs: Antihypertensive drugs.* Significantly higher in group2 versus group1 at (p<0.05).
Table 1: Baseline demographics and clinical data.
Fatty liver grading on ultrasound
The fatty liver grading on ultrasound also showed non-significant difference at baseline (p=0.757). In both groups there was a significant regression in fatty liver grading after treatment when compared to patient’s fatty liver grading before treatment (p =0.001). When comparing of regression in fatty liver grading in both groups although the regression was slightly higher in group2 but there was no significant difference between them. These results are shown in Table 2.
| Ultrasonography | Group 1 | Group 2 | P-value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Pre | Grade 0 | 0 | 0 | 0 | 0 | 0.757 |
| Grade 1 | 19 | 31.7 | 21 | 35 | ||
| Grade 2 | 31 | 51.7 | 27 | 45 | ||
| Grade 3 | 10 | 16.7 | 12 | 20 | ||
| Post | Grade 0 | 19 | 31.7 | 20 | 33.3 | 0.659 |
| Grade 1 | 15 | 25 | 19 | 31.7 | ||
| Grade 2 | 20 | 33.3 | 18 | 30 | ||
| Grade 3 | 6 | 10 | 3 | 5 | ||
| P-value | 0.001 | 0.001 | ||||
N: number of patients. pre: pretreatment; post: post treatment
Table 2: The effect of vildagliptin versus vildagliptin/metformin on fatty liver grading on ultrasound.
BMI and biological parameters
The effects of both treatments on BMI and biological parameters are shown in Table 3. The obtained data showed that in both groups there was significant decrease in BMI, fasting and post prandial serum glucose, HBA1c, ALT &AST, TC, TG, LDL-C level, fasting insulin, HOMA-IR, ferritin beside a significant increase in HDL-C and adiponectin level while there was slight increase in blood urea and serum creatinine level after treatment compared to baseline in both groups but the elevation was not significant (Table 3).
| Parameters | Group1 | Group2 | ||||
|---|---|---|---|---|---|---|
| Pre treatment | Post treatment | P value | Pre treatment | Post treatment | P value | |
| BMI (Kg/m2) | 31.50 ± 2.87 | 29.74 ± 2.74 | 0.001a | 31.95 ± 3.66 | 28.45 ± 3.37 | 0.001a,b |
| FSG (mg/dl) | 160.82 ± 34.00 | 104.87 ± 9.95 | 0.001a | 161.93 ± 29.82 | 93.52 ± 12.71 | 0.001a,b |
| PPSG (mg/dl) | 318.27 ± 98.13 | 182.72 ± 13.81 | 0.001a | 323.38 ± 102.32 | 162.18 ± 22.41 | 0.001a,b |
| HbA1c (%) | 7.02 ± 0.25 | 6.49 ± 0.31 | 0.001a | 8.05 ± 0.46 | 6.96 ± 0.44 | 0.001a,b |
| BUN (mg/dl) | 29.07 ± 5.56 | 30.27 ± 5.47 | 0.236 | 29.33 ± 5.35 | 30.13 ± 5.14 | 0.405 |
| S.Cr. (mg/dl) | 0.63 ± 0.20 | 0.69 ± 0.19 | 0.096 | 0.61 ± 0.24 | 0.69 ± 0.21 | 0.059 |
| AST (IU/L) | 30.82 ± 6.67 | 23.62 ± 5.58 | 0.001a | 31.69 ± 5.51 | 21.58 ± 4.31 | 0.001a,b |
| ALT (IU/L) | 38.58 ± 11.26 | 29.68 ± 5.43 | 0.001a | 39.95 ± 11.05 | 26.85 ± 6.74 | 0.001a,b |
| TC (mg/dl) | 183.73 ± 17.85 | 169.55 ± 16.65 | 0.001a | 182.60 ± 17.41 | 156.27 ± 18.79 | 0.001a,b |
| TG (mg/dl) | 146.63 ± 18.56 | 133.34 ± 17.79 | 0.001a | 146.25 ± 20.46 | 130.93 ± 17.6 | 0.001a |
| LDL-C (mg/dl) | 111.44 ± 24.59 | 97.26 ± 23.31 | 0.002a | 113.73 ± 26.03 | 75.51 ± 22.34 | 0.001a,b |
| HDL-C (mg/dl) | 42.97 ± 17.01 | 55.82 ± 11.85 | 0.001a | 40.30 ± 18.30 | 56.02 ± 11.17 | 0.001a |
| Insulin (µIU/ml) | 13.30 ± 4.89 | 11.09 ± 3.71 | 0.006a | 13.22 ± 5.08 | 8.75 ± 3.56 | 0.001a,b |
| HOMA-IR | 5.29 ± 2.23 | 3.95 ± 1.26 | 0.001a | 5.25 ± 2.09 | 2.55 ± 1.11 | 0.001a,b |
| Adiponectin (µg/ml) | 7.15 ± 2.35 | 10.08 ± 4.15 | 0.001a | 6.79 ± 2.50 | 10.44 ± 4.68 | 0.001a |
| Ferritin (ng/ml) | 178.71 ± 84.77 | 139.93 ± 68.95 | 0.007a | 188.63 ± 83.19 | 117.03 ± 52.08 | 0.001a,b |
Data are presented as mean ± SD. Group1: patients with type 2 diabetes mellitus and NAFLD received Vildagliptin in a dose of 50 mg twice daily; Group2: patients with type 2 diabetes mellitus and NAFLD received Vildagliptin/ Metformin in a dose of 50 mg/ 1000 mg twice daily. BMI: body mass index; FSG: fasting serum glucose; PPSG: post prandial serum glucose; BUN: blood urea nitrogen; S.Cr.: serum creatinine; HbA1c: hemoglobin A1c (glycated hemoglobin); AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TC: Total cholesterol; TG: Triglycerides; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol; HOMA-IR: Homeostatic model assessment of insulin resistance. a: significant in post treatment versus pretreatment at P<0.05; b: significant in group2 versus group1 at P<0.05.
Table 3: Effect of vildagliptin /metformin combination versus vildagliptin alone on selected parameters after twelve months of treatment
When we compared the efficacy of vildagliptin alone versus vildagliptin/metformin combination the following results were found: After twelve months the reduction in BMI (–1.76 Kg/m2 vs –3.5 Kg/m2, respectively with P = 0.024),FSG (–55.95 mg/dl vs –68.41 mg/dl, respectively with P = 0.001) , PPSG (–135.55 mg/ dl vs –161.2 mg/dl, respectively with P = 0.001), HBA1c (–0.53% vs –1.09%, respectively with P = 0.001) , ALT (–8.9 IU/L vs –13.1 IU/L, respectively with P = 0.013) , AST (–7.2 IU/L vs –10.11 IU/L, respectively with P = 0.027) , fasting insulin (–2.21 μIU/ml vs –4.47μIU/ml, respectively with P = 0.001) , HOMA-IR (–1.34 vs –2.7, respectively with P = 0.001), ferritin level (–38.78 ng/ml vs –71.6 ng/ml, respectively with P = 0.042), TC (–14.18 mg/dl vs –26.33 mg/dl, respectively with P = 0.001) and LDL-C (–14.18 mg/ dl vs –38.22 mg/dl, respectively with P = 0.001) was significantly greater in group 2 than in group1.
There was no significant difference between both groups in reduction of TG (–13.29 mg/dl vs –15.32 mg/dl, with P ˃0.05), blood urea and serum creatinine level (p=0.891) and (p=1.00) respectively.
Also, elevation of HDL-C (+12.85 mg/dl vs +15.72 mg/dl, with P ˃0.05) and adiponectin level values in group 2 was slightly greater but not significant than the increase in group1 (+2.93 μg/ml vs +3.65 μg/ml in group 2, with P ˃0.05).
In both groups a correlation study between NAFLD grading and measured parameters after treatment revealed a significant positive correlation between NAFLD grading and BMI, HbA1c, fasting insulin, HOMA-IR, ALT, AST and TG. However, a significant negative correlation was observed between NAFLD grading and HDL and adiponectin as shown in Table 4.
| NAFLD grading | Group 1 | Group 2 | ||
|---|---|---|---|---|
| r | P | r | P | |
| BMI (Kg/m2) | 0.569 | 0.001* | 0.311 | 0.016* |
| FSG (mg/dl) | 0.065 | 0.620 | -0.092 | 0.487 |
| Fasting insulin (µIU/ml) | 0.567 | 0.001* | 0.637 | 0.001* |
| HOMA - IR | 0.679 | 0.001* | 0.647 | 0.001* |
| PPSG (mg/dl) | 0.090 | 0.496 | 0.126 | 0.336 |
| HbA1c (%) | 0.681 | 0.001* | 0.311 | 0.016* |
| ALT (IU/L) | 0.430 | 0.001* | 0.612 | 0.001* |
| AST (IU/L) | 0.349 | 0.006* | 0.444 | 0.001* |
| TC (mg/dl) | 0.084 | 0.524 | 0.119 | 0.366 |
| TG (mg/dl) | 0.652 | 0.001* | 0.682 | 0.001* |
| LDL-C (mg/dl) | 0.020 | 0.876 | 0.138 | 0.292 |
| HDL -C(mg/dl) | -0.460 | 0.001* | -0.696 | 0.001* |
| Adiponectin (µg/ml) | -0.630 | 0.001* | -0.496 | 0.001* |
| Ferritin (ng/ml) | 0.189 | 0.149 | 0.144 | 0.272 |
Data are presented as r (Pearson's correlation coefficient. BMI: body mass index; FSG: fasting serum glucose; PPSG: post prandial serum glucose; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TC: Total cholesterol; TG: Triglycerides; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol; HOMA-IR: Homeostatic model assessment of insulin resistance; HbA1c: hemoglobin A1c (glycated hemoglobin). *: significant correlation at p<0.05. -: negative correlation.
Table 4: Correlation study between NAFLD grading and measured parameters after treatment in both groups.
Univariate and multivariate analysis for the parameters affecting the improvement in NAFLD grading in both studied groups are shown in Figure 2 and Table 5. Improvement in NAFLD grading in both groups was affected by change in BMI, HbA1c, fasting insulin, HOMA-IR, TG and adiponectin. The most independent factors affecting improvement in NAFLD were change in TG, adiponectin, fasting insulin, HOMA-IR and BMI. As, the greater the reduction in post treatment TG level, serum fasting insulin, HOMA-IR and BMI the better the improvement in NAFLD grading. While, the greater the increase in post treatment level of serum adiponectin the better the improvement in NAFLD grading.
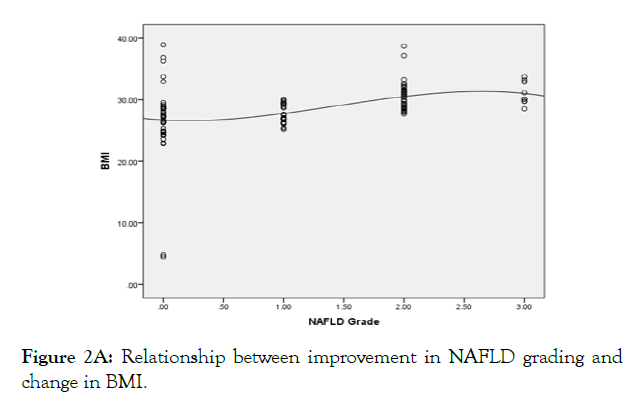
Figure 2A: Relationship between improvement in NAFLD grading and change in BMI.
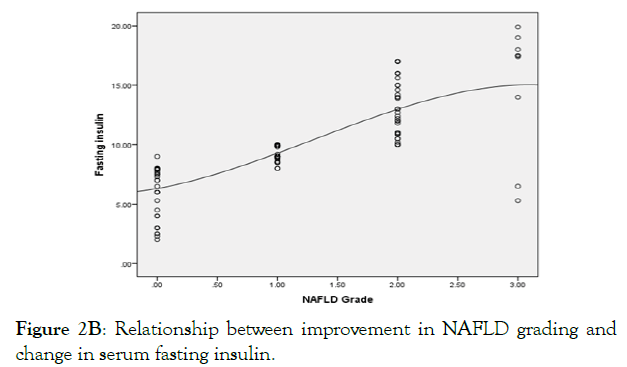
Figure 2B: Relationship between improvement in NAFLD grading and change in serum fasting insulin.
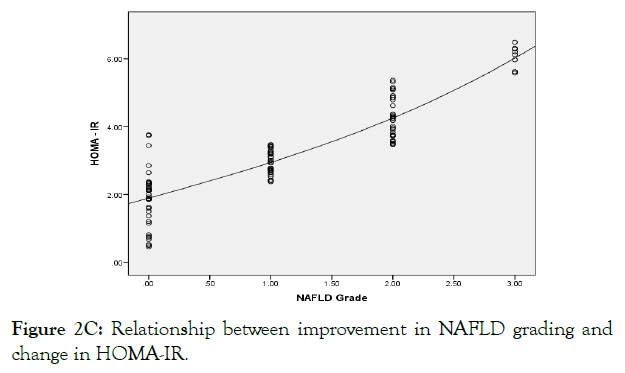
Figure 2C: Relationship between improvement in NAFLD grading and change in HOMA-IR.
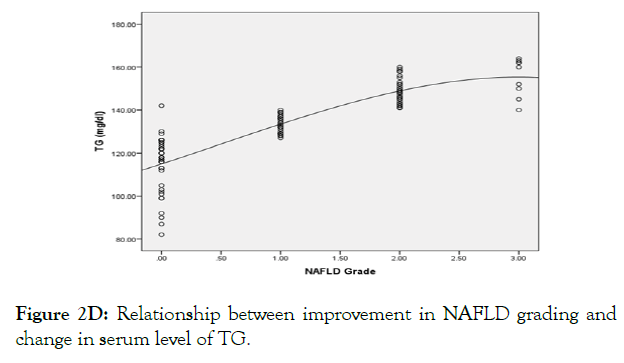
Figure 2D: Relationship between improvement in NAFLD grading and change in serum level of TG.
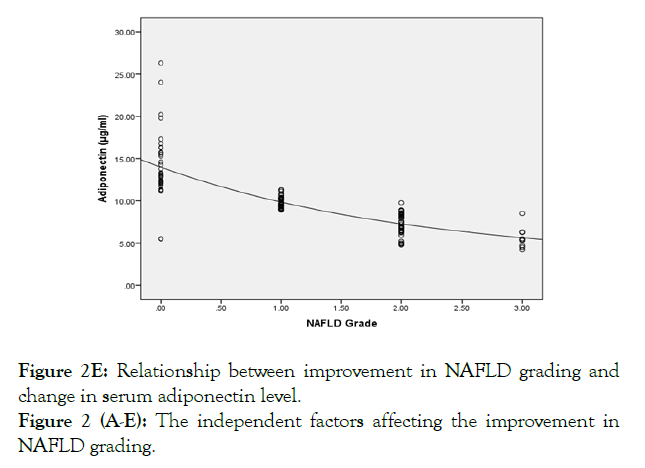
Figure 2E: Relationship between improvement in NAFLD grading and change in serum adiponectin level.
Figure 2 (A-E): The independent factors affecting the improvement in NAFLD grading.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| BMI | 0.489 (0.229 – 0.658) | 0.027* | 0.519 (0.319 – 0.759) | 0.037* |
| Fasting insulin | 0.624 (0.198 – 0.905) | 0.032* | 0.725 (0.367 – 0.926) | 0.021* |
| HOMA - IR | 0.514 (0.185 – 0.873) | 0.036* | 0.625 (0.237 – 0.805) | 0.028* |
| HbA1c | 0.741 (0.369 – 0.859) | 0.003* | 4.528 (0.199 – 9.748) | 0.208 |
| ALT | 0.496 (0.236 – 1.754) | 0.134 | ||
| AST | 0.854 (0.206 – 3.627) | 0.208 | ||
| TG | 0.521 (0.264 – 0.854) | 0.001* | 0.745 (0.299 – 0.958) | 0.005* |
| HDL-C | 2.307 (0.367 – 8.523) | 0.317 | ||
| Adiponectin | 2.064 (1.299 – 6.958) | 0.001* | 3.658 (1.597 – 9.632) | 0.014* |
OR: Odds ratio; C.I: Confidence interval. *: Statistically significant at p<0.05. All variables with p<0.05 was included in the multivariate analysis. BMI: body mass index; HOMA-IR: Homeostatic model assessment of insulin resistance; HbA1c: hemoglobin A1c (glycated hemoglobin); AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TG: Triglycerides; HDL-C: High density lipoprotein cholesterol.
Table 5: Univariate and multivariate analysis for the parameters affecting the improvement in NAFLD grading in both groups.
Safety and tolerability
Vildagliptin was well tolerated, and there were no meaningful differences between group1and group2 in the overall adverse events (AEs) profile except for GIT disturbance which was highly significant in group2 versus group1. There was no discontinuation of both treatments due to AEs. Adverse events occurred in 6 patients in the group1 vs 10 patients in group2. Most frequent AEs in the categories of; respiratory tract infection (one patient in each group had upper respiratory tract infection), back pain (one vs two patients), headache (two vs none of the patients in group2) and dizziness (two vs one patient). Mild hypoglycemia was reported (none of the patients in group1 vs one patient in group2). Gastrointestinal disturbances in the form of bloating and abdominal pain were reported in 5 patients in group2 while none of the patients were reported in group1.
To the best of our knowledge, this is the first clinical study with long follow up period to study the effect of vildagliptin versus vildagliptin / Metformin combination on fatty liver disease. The present study included a larger number of patients with NAFLD as compared to the previous studies of vildagliptin on NAFLD patients. In the present study there was a significant improvement in the grading of NAFLD on ultrasonography in both treatment groups. These results are supported by previous study carried out by Hussain et al. [11] who found that vildagliptin induced regression of fatty liver by more than 60% which was significant [11]. Our results were also in line with Macauley et al. [14] who reported that vildagliptin plus metformin brings about a clinically significant decrease in hepatic triglyceride levels during 6 months of therapy [14] with intensive research we did not find any study addressed the effect of vildagliptin or vildaglptin/metformin combination on fatty liver disease with outcome against our findings.
The univariate and multivariate analysis in our study showed that reduction in BMI was independent factor affecting improvement in NAFLD grading. The mainstay of NAFLD management is currently achieving good glycemic control and optimizing weight loss as both are pivotal to restrict disease progression [15]. In the present study there was a significant reduction in BMI after twelve-month treatment in both groups. Similar results were obtained by Hussain et al. [11] who reported that vildagliptin causes a significant reduction in BMI after 12 weeks’ treatment [11] also, Macauley et al. [14] found that treatment with vildagliptin added to metformin resulted in weight loss vs baseline [14]. On the other hand Schweitzer et al. [16] reported that body weight did not change during the 1-year treatment with vildagliptin and Filozof et al. [17] study that compared vildagliptin and gliclazide as add-on to metformin over 52 weeks reported no reduction in body weight with vildagliptin [16,17]. This confliction in results with above authors may be explained by the difference in methodology and studied population. The improvement in fatty liver grading due to reduction in BMI can be understood in the view of improvement in TG metabolism and insulin resistance with weight reduction. So weight loss improves liver histology [11,15].
In our study there was a significant improvement in HbA1c in both groups. The improvement in HbA1c was one of the independent factors affecting the regression of NAFLD grading in univariate analysis. A number of previous studies have reported the effect of DDP-4 inhibitors on HbA1c in NAFLD patients; these studies have showed that DPP-4 inhibitors were safe and efficacious treatment for hyperglycemia in those patients with diabetes and NAFLD either alone or in combination with metformin [18-23]. Good glycemic control can lead to improvement in lipid metabolism and insulin sensitivity with subsequent improvement in fatty liver disease [5].
Insulin resistance as measured by fasting insulin level and HOMA-IR was significantly improved after treatment with vildagliptin and vildagliptin/metformin combination in our studied patients and this improvement was shown to be significantly independent factor in regression of fatty liver disease grading using univariate and multivariate analysis. Our study was in line with another study by Pratley et al. [24] who found that insulin resistance was attenuated with vildagliptin treatment, as evidenced by a significant decrease in HOMA-IR vs. baseline and vs. placebo [24]. Similarly Kitao et al. [25] reported that HOMA-IR improved following 12 weeks of treatment with vildagliptin/metformin combination [25]. Our results could be explained by the effect of vildagliptin on intact glucagon-like peptide-1(GLP-1) level that increases by inhibition of DPP-4 enzyme so improves α-cell glucose sensitivity and promote glucose-dependent insulin secretion from pancreatic β cells, whereas metformin, rises simultaneously GLP-1 synthesis or induces peroxisome proliferator-activated receptors( PPAR-α/ PPAR-γ-) dependent islet gene expression and incretin receptor responsiveness. Some studies indicate that metformin potentiates the effect of insulin on glucose transport at a site beyond insulin receptor binding and phosphorylation so reduces both peripheral and hepatic insulin resistance [26,27]. It was reported that there was correlation between insulin resistance as measured by the homeostatic model assessment and NAFLD identified by an echogenic liver using ultrasonography which suggest that any improvement in insulin resistance will exhibits beneficial effect in NAFLD patients [28]. However, our results was in contrast with Rosenstock et al. [29] who reported that the reduction in HOMAIR after12-weeks treatment with vildagliptin was not statistically significant compared to placebo and with Bo Ahrén et al. [30] who found that fasting insulin and HOMA-IR did not change significantly after treatment with vildagliptin plus metformin in type2 diabetic patients [29,30]. This contrast could be explained by the difference in the study duration, methodology and studied population.
Our study found a significant improvement in TG level after treatment in both groups which was shown to be the most significant independent factor affecting the improvement in fatty liver grading using univariate and multivariate analysis. These findings were in agreement with the work of Hussain et al. [11] which reported that there was significant improvement in lipid profile after twelve weeks of vildagliptin treatment [11]. Also, there is a prospective randomized study by Tani et al. [31] showed that 8-weeks treatment with vildagliptin for type 2 diabetes improved TG metabolism [31]. In the same way Xiea et al. [32] reported that vildagliptin showed a favorable fasting lipid profile that was associated with modest weight loss [32]. This finding was also in consistent with another study by Derosaa et al. [33] which found that vildagliptin plus metformin proved to be more effective than glimepiride plus metformin in reducing post-prandial lipemia [33]. The change in lipid profiles after vildagliptin therapy could be related to reduce fasting lipolysis in fat cells, and increase postprandial lipolysis in adipose tissue as well as postprandial fat oxidation in muscle, the liver, and pancreas, influence of vildagliptin on post-prandial lipemia could reflect incretin-mediated changes in pancreatic hormone secretion, an improved metabolic state and reduced insulin resistance secondary to improved islet function, incretin mediated effects to slow gastric emptying, or direct effects of GLP- 1 and/or glucose-dependent insulinotropic polypeptide (GIP) on lipoprotein metabolism [3, 34]. On the other hand, our finding is in contraindication with another previously reported clinical study by Fonseca et al. [35] which showed that vildagliptin when added to insulin had no statistically significant effect on fasting triacylglycerol, HDL-cholesterol or very low density lipoprotein cholesterol [35]. This contrariness can be attributed to the short duration of the previous study which was only 24 weeks compared to our study which lasted for 12 months.
Studies showed that low level of adiponectin may play a role in the pathogenesis of NAFLD by decreasing the oxidation of fatty acids in the liver, so improvement in adiponectin level could improve fatty liver oxidation with improvement in NAFLD beside the positive effect of adiponectin on insulin resistance [36]. Also, adiponectin levels have been found to be significantly lower in subjects with NAFLD or NASH compared with controls [36]. Our results indicate that serum adiponectin level significantly increased in both treatment groups which was also a significant independent factor affecting the improvement of NAFLD grading as shown in univariate and multivariate analysis. These results were further confirmed by a meta-analysis of ten randomized controlled trials by Liu et al. [37] which found that vildagliptin treatment significantly elevates adiponectin levels [37]. These findings were also in agreement with another study by Kitao et al. [25] which found that the addition of vildagliptin to metformin significantly improved serum adiponectin levels compared with high-dose metformin and were also in line with Schiapaccassa et al. [38] who found that vildagliptin treatment significantly increased adiponectin compared to metformin alone. This finding is compatible with the mechanism by which vildagliptin may counteract inflammation and decrease oxidative stress or visceral fat since increase of systemic and/or local oxidative stress reduces adiponectin production, reduction of body weight and visceral fat also result in the elevation of serum adiponectin level [25,32,39].
The limitations of this study were that ultrasonography was the method to evaluate NAFLD and liver biopsy which is the gold standard method for the diagnosis of NAFLD was not used due to its invasiveness and low acceptance rate in patients of simple NAFLD. Also the study was a single center study and non-diabetic patients were not included in the study. So we recommend a multicenter study for evaluation of different population groups including non-diabetic patients with fatty liver disease.
In conclusion, treatment of individuals with type2 diabetes mellitus and NAFLD with vildagliptin or vildagliptin/metformin combination showed a significant regression of NAFLD grading using ultrasonography. These findings could be attributed to reduction in BMI, improvement in serum insulin and HOMA-IR, reduction of serum TG and elevation of serum adiponectin level.
Acknowledgements: The authors are thankful to the members of departments of clinical pathology & radiology of Tanta University Educational Universal Hospital, for their cooperation.
Declarations of interest: The authors declare that they have no conflict of interest.
Funding: This study received no funding.
Ethics statement: All participants gave their written informed consent. The study was approved by Tanta University Research Ethical Committee, with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and was registered on the database of clinical trials (ClinicalTrials.gov, ID no. NCT03925701).
Citation: Aya Gamal M, Gamal AA, Ibrahim OM, Abdelraouf YM (2021) Effect of Vildagliptin versus Vildagliptin plus Metformin on Nonalcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus. J Diabetes Metab. 12:890.
Received: 18-May-2021 Published: 27-Aug-2021, DOI: 10.35248/2155-6156.21.12.890
Copyright: © 2021 Aya Gamal M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.