Research Article - (2020) Volume 11, Issue 5
Objectives: The purpose of this study was to investigate the efficacy and safety of teneligliptin, a completely unique and highly selective DPP-4 inhibitor in type 2 diabetes mellitus (T2DM) patients having dyslipidemia who are inadequately controlled by relevant conventional therapy in India.
Methods: Study protocol was approved by Institutional Ethics Committee. Diabetic patients having dyslipidemia (male/female) were randomized to receive treatments in two groups, namely conventional therapy [treatment (A)] and add on teneligliptin 20 mg with conventional therapy [treatment (B)] for 24 weeks. Predesigned case report form (CRF) was used to collect information from the prescribing physicians regarding the efficacy and safety of teneligliptin. Efficacy variables included change in serum glycaemic, lipid, and cytokines (IL-6, TNF-α and adiponectin) levels from baseline to week 24. Treatment-emergent adverse events (TEAEs) were also assessed.
Results: A complete of 120 T2DM patient having dyslipidemia were analysed using graph pad prism. Teneligliptin, as add on therapy to conventional therapy significantly reduced serum lipid profile (TC, TG, and LDL) as well as glycaemic parameters (HbA1c, FBG, and PPBG) along with significant rise in serum adiponectin levels as compared to conventional therapy.
Conclusion: Add- on therapy with teneligliptin was found better option over convetional therapy in term of significantly reduced glycemic as well as lipid profile. Further, it was found safe and well tolerated in T2DM patients having dyslipidemia.
Teneligliptin; DDP-4 inhibitor; Metformin; Total cholesterol; Glycated haemoglobin; Adiponectin; Type 2 diabetes mellitus; Dyslipidemia
NCD: Non-Communicable Disease; IDF: International Diabetes Federation; T2DM: Type 2 Diabetes Mellitus; DPP-4: Dipeptidyl Peptidase-4; GLP-1: Glucagon-like Peptide-1; HbA1c: Glycated Haemoglobin; IEC: Institutional Ethics Committee; DCGI: Drugs Controller General of India; BMI: Body Mass Index; FBG: Fasting Plasma Glucose; PPBG: Post Prandial Blood Glucose; AE: Adverse Event; SAP: Sample Analysis Plan; SD: Standard Deviation
Diabetes mellitus (DM) is found to be related to alterations in lipid metabolism in term of high total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL) and low high density lipoprotein (HDL), that ends up in dyslipidemia and worsens the prognosis of diabetic patients having atherosclerosis and Cardiovascular disorder(CVD) [1]. The International Diabetes Federation (IDF) estimates the whole number of diabetic subjects is to rise to 69.9 million USA, China, and India by the year 2025 [2]. The population of diabetes is rapidly growing because of the expansion of population, urbanisation, ageing, and increasing prevalence of obesity and morbidity [3].
Dyslipidemia, a long-time established risk factor for CVD has effect on 50% of diabetes patients as compared to non-diabetic population [4]. In DM patient’s insulin deficiency or resistance activates intracellular hormone-sensitive lipase which increases the discharge of non-esterified fatty acids (NEFA) from triglycerides that’s stored in centrally distributed adipose tissue. High circulating levels of NEFA also increase hepatic triglyceride production [5,6]. Lower levels of adiponectin are considered as an independent risk factor for developing Type 2 DM, dyslipidemia, and cardiovascular diseases [7].
A recent statement from one amongst the meta analysis report for the standards of medical care in Diabetes by the American Diabetes Association (ADA) has recommended that initially treatment with metformin as monotherapy after inadequate life style modification, followed by sulfonylurea, thiazolidinedione, Dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose co-transporter 2 inhibitors (SGLT2-i), glucagon like peptide 1 (GLP 1) receptor agonist, or insulin alone or in combination [8]. However, it’s still difficult to search out an antidiabetic agent with long-term glucose control, minimal hypoglycaemia, no weight gain with affordable price. The optimum treatment with antidiabetic drugs to get fair glycaemic control should go hand-in-hand with lipid-lowering drugs [9]. In diabetic patients having dyslipidemia, in keeping with current guideline, statins are preferred as they are well tolerated, efficacious and don’t adversely affect glycaemic control [10].
DPP-4 inhibitors are considered as a cornerstone within the management of T2DM due to their robust efficacy and favourable tolerability profile [11]. Our previous study has demonstrated teneligliptin as add on therapy showing better glycemic control as compared to conventional therapy in Indian patients having T2DM [12]. As there is no future study conducted on add on therapy of teneligliptin in diabetic dyslipidemic patients, our study was designed to evaluate efficacy, safety, and tolerability treatment which can lead to improvement in the effectiveness of standard therapy in diabetic patients having dyslipidemia in India. Currently only few reports are available on the role of inflammatory biomarkers in type 2 diabetes and folks with impaired lipid metabolism in Indian population. During within this manuscript, we have also tried to incorporate the role of inflammatory regulators (IL 6, TNF ά and adiponectin) with respect to add on teneligliptin therapy in type 2 diabetic patients having dyslipidemia in Indian population.
Ethics approval
The protocol of clinical study, informed consent form and relevant essential documents were approved by Institutional Ethics Committee (IEC); Safety, Health and welfare Ethics committee, registered under Drug Controller General of India (DCGI). The study was conducted according to the Ethical principles of Declaration of Helsinki; Good Clinical Practices guidelines issued by the Central Drugs Standard Control Organization (CDSCO), Indian Councils of Medical Research (ICMR).
Study design and procedure
A prospective, randomized, open label, study to assess the efficacy and safety of teneligliptin as an add-on therapy to conventional treatment in T2DM patients having dyslipidemia. This study was conducted at Jivraj Mehta Hospital and Bakeri Medical Research Centre, Ahmedabad.
Eligibility criteria
Study protocol was clearly defined for the patients and informed consent was obtained from all patients before participation. The study included male and female patients with T2DM having dyslipidemia, aged >18 years, HbA1c levels of >7.0%, and body mass index (BMI) of 20.0–35.0 kg/m2 (both inclusive). Patients were excluded if they had serious disease such as kidney, liver, and cerebral stroke, history of severe heart disease or arrhythmias, taking DPP-4 inhibitor other than teneligliptin, taking statin other than atorvastatin and on insulin therapy, pregnant, pregnant, lactating mother, malignancy, any acute illness and history of alcohol and tobacco use.
Intervention
Eligible patients were randomized in 1:1 ratio to receive either metformin (500 mg/day) + glimepiride (2 mg/day) and atorvastatin (20 mg/day) (treatment A) or metformin (500 mg/day) + glimepiride (2 mg/day), atorvastatin (20 mg/day) and add on teneligliptin (20 mg/day) (Treatment B) for 24 weeks. Patient’s demographics data, physical and clinical examination, laboratory assessments were documented in predesigned case report form (CRF).
Biochemical parameters: Lipid profile including serum Total cholesterol (TC), Triglyceride (TG), Low density lipoprotein (LDL), High density lipoprotein (HDL) and Glycemic parameters including serum glycated haemoglobin (HbA1c), fasting blood glucose (FBG) and post prandial blood glucose (PPBG) levels and Inflammatory cytokine levels IL6, TNF-α, and adiponectin levels were measured at baseline and at the end of 24 weeks in both the treatment groups.
Methodology
Serum cholesterol was estimated by cholesterol oxidase method from ROCHE on COBAS INTEGRA 400 WITH Exteranal quality control (EQAS) from BIO-RAD laboratories (USA) internal quality control from ROCHE diagnostic. Serum LDL and HDL were tested by direct non-immunological method on COBAS INTEGRA 400PLUS. Serum Triglyceride was tested by lipase glycerol method. Values of serum lipids were entered into the computer and computer analysis of the data was obtained.
Cytokines estimation
From each subject, serum was collected and stored at -80 ºC until further analysis. Serum IL-6 and TNF-α levels were measured using the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (KRISHGEN Biosytems, Mumbai). The assay sensitivity ranges of KRISHGEN Biosytems kits were 3.12-200 pg/ml for IL-6 and 6.8-500 pg/ml for TNF-α in serum samples. The ELISA kits were validated with inter- and intra-assay precision. Adiponectin level was measured using the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (KINESIS Dx). The assay sensitivity ranges of KINESIS Dx kit was 2-34 μg/ml adiponectin, for adiponectin in serum samples. The ELISA kit was validated with inter- and intraassay precision. The (IL-6, TNF- α, and adiponectin) cytokines
Efficacy and safety end points
The primary efficacy end point was the change in glycemic and lipid parameter from baseline to 24 weeks. Secondary efficacy endpoints include change in inflammatory (IL-6, TNF- α, and adiponectin) cytokine levels from baseline to 24 weeks. During the clinical study period, we monitored possible adverse events (AEs), laboratory values, vital sign and physical examination results. Safety aspects were measured by recording AEs including symptomatic assessment by Naranjo causality scale for adverse events [13]. The incidence of AE in terms of number per patient was calculated based on the number of events, the number of patients and the total observation period.
Sample size and Statistical analysis
The primary end point, difference in mean HbA1c from baseline to 24 week was assumed 0.5% and also the standard deviation (SD) of 0.9% for each treatment group. Based on a power of 80% and a type I error rate of alpha=0.05 (2-tailed), a sample size of at least 60 patients per group was required to detect a clinically significant difference between both the groups [14]. Categorical data was presented as absolute number/percentage of patients while quantitative data was presented as mean ± standard deviation (SD). Within group comparison was performed using paired t-test based on the distribution of data. Unpaired t-test was used to analyse the quantitative data for between group comparisons. Missing data was handled using Last observation carried forward (LOCF) method. P value of less than 0.05 was considered as statistical significant difference. Data were calculated using Graph Pad prism version 5.0.
A flow chart is presented showing the disposition of participants throughout the study (Figure 1). Out of 159 screened patients, 132 eligible patients were randomized during this clinical study. Treatment A included 61 patients and treatment B included 63 patients. As per sample analysis plan (SAP), sixty patients in each group were analysed to detect a clinically significant difference between both the groups Both the groups demographic and clinical characteristic parameters at baseline was mentioned in Table 1.
Table 1: Demographic and clinical characteristic.
| Characteristic | Treatment A (N=60) | Treatment B (N=60 |
|---|---|---|
| Demographic | ||
| Gender(Male/Female) | 31/29 | 34/26 |
| Age (year) | 48.56 ± 8.66 | 50.41 ± 7.27 |
| Height (cm) | 159.9 ± 10.47 | 164.21 ± 8.80 |
| Body Weight (kg) | 69.06 ± 17.79 | 76.90 ± 13.40 |
| Body mass index (BMI) | 25.85 ± 3.84 | 28.67 ± 4.76 |
| Disease duration (year) | 4.56 ± 1.61 | 4.86 ± 1.50 |
| Waist (cm) | 92.93 ± 8.86 | 95.72 ± 10.07 |
| Hip (cm) | 98.63 ± 10.33 | 102.33 ± 11.33 |
| Pulse /min | 87.16 ± 13.96 | 85.96 ± 13.01 |
| Systolic blood pressure (mmHg) | 132.9 ± 18.70 | 132.97 ± 18.85 |
| Diastolic blood pressure (mmHg) | 80.91 ± 8.66 | 80.28 ± 10.19 |
Values are expressed in terms of Mean ± SD. N: Number of patient, SD: Standard Deviation. Treatment A: Conventional treatment and Treatment B: Add on teneligliptin with conventional treatment.
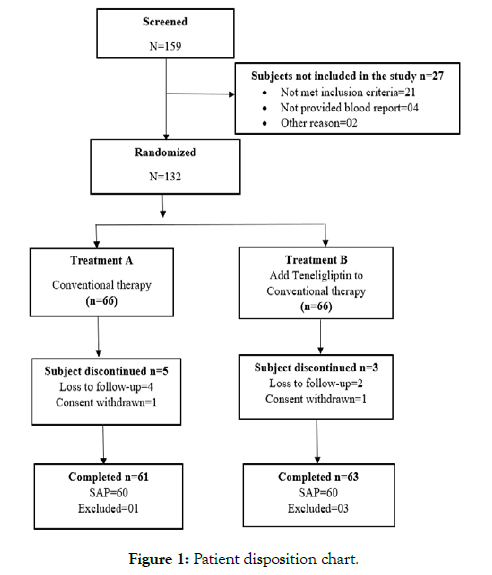
Figure 1: Patient disposition chart.
Glycaemic parameters: HbA1c level was found comparable in both the treatment groups at the baseline. However, there was gradual reduction in HbA1c over the period of 24 weeks in both the treatment groups. Between groups comparison showed significant reduction (p<0.05) in HbA1c in treatment B as compared to treatment A (Figure 2).
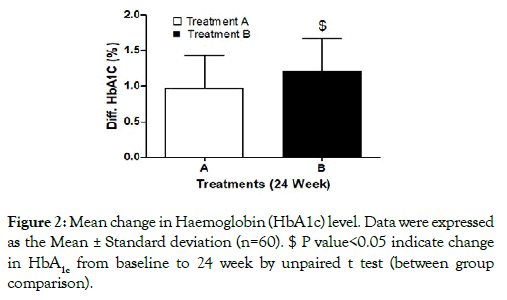
Figure 2: Mean change in Haemoglobin (HbA1c) level. Data were expressed as the Mean ± Standard deviation (n=60). $ P value<0.05 indicate change in HbA1c from baseline to 24 week by unpaired t test (between group comparison).
Blood glucose levels (FBG and PPBG) were found comparable in both the treatment groups at the baseline. However, there were significant reduction in FBG and PPBG levels over a period of 24 weeks in both the treatment groups. However, reduction in glycaemic parameters (HbA1c, FBG and PPBG) was statistically significant in treatment B as compared to treatment A after 24 weeks (Table 2). Reduction in mean change of HbA c was 0.96 ± 0.46 and 1.20 ±0.45 in in treatment A & B respectively. Reduction in mean changes in FBG and PPBG levels were 23.56 ± 8.40 and 28.89 ± 9.17 in treatment A as compared to 32.62 ± 11.45 and 36.62 ± 9.92in treatment B respectively.
Table 2: Mean change in blood lipid and glycemic levels from baseline to 24 weeks after study drug treatments.
| Parameters | Treatment A(N=60) | Treatment B(N=60) |
|---|---|---|
| Total cholesterol (TC) | ||
| Baseline (TC) | 218.08 ± 20.15 | 219.00 ± 14.18 |
| End of 24 weeks | 176.78 ± 13.22* | 166.90 ± 19.56* |
| Change in TC | 41.22 ± 18.57 (18.90%) | 52.10 ± 20.92# (23.78%) |
| Triglyceride (TG) | ||
| Baseline (TG) | 190.88 ± 18.62 | 197.36 ± 18.00 |
| End of 24 weeks (TG) | 160.13 ± 18.60* | 157.88 ± 17.30* |
| Change in TG | 30.75 ± 11.56 (16.10%) | 39.48 ± 15.25# (20.00%) |
| High density lipoprotein (HDL) | ||
| Baseline (HDL) | 36.44 ± 4.38 | 37.32 ± 5.47 |
| End of 24 weeks (HDL) | 39.08 ± 4.61* | 40.56 ± 5.53* |
| Change in HDL | 2.64 ± 0.98 (7.24 %) | 3.24 ± 1.08 (8.68%) |
| Low density lipoprotein (LDL) | ||
| Baseline (LDL) | 158.54 ± 13.15 | 155.90 ± 17.39 |
| End of 24 weeks (LDL) | 113.76 ± 17.03* | 103.09 ± 17.76* |
| Change in LDL | 44.78 ± 11.61 (28.24%) | 52.80 ± 15.77# (34.06%) |
| TC/HDL ratio (atherogenic Index) | ||
| Baseline | 6.07 ± 0.94 | 6.06 ± 1.05 |
| End of 24 weeks | 4.59 ± 0.69* | 4.17 ± 0.66* |
| Change in ration TC/HDL | 1.48 ± 0.53 (24.38%) | 1.82 ± 0.76 (30.03%) |
| Glycated heamglobin (HbA1c) | ||
| Baseline | 9.63 ± 1.24 | 9.45 ± 1.27 |
| End of 24 weeks | 8.67 ± 1.29* | 8.24 ± 1.21* |
| Change in HbA1c | 0.96 ± 0.46 (9.96%) | 1.20 ± 0.45$ (12.69%) |
| Fasting blood glucose (FBG) | ||
| Baseline | 153.54 ± 20.95 | 157.53 ± 19.82 |
| End of 24 weeks | 129.98 ± 16.65* | 124.91 ± 18.54* |
| Change in FBG | 23.56 ± 8.40 (15.34%) | 32.62 ± 11.45$ (20.70%) |
| Post prandial blood glucose (PPBG) | ||
| Baseline | 248.46 ± 27.35 | 252.82 ± 27.58 |
| End of 24 weeks | 219.56 ± 27.67* | 216.19 ± 25.92* |
| Change in PPBG | 28.89 ± 9.17 (11.62%) | 36.62 ± 9.92$ (14.48%) |
| Inflammatory cytokines | ||
| IL 6 (pg/ml) | ||
| Baseline | 8.027 ± 0.92 | 7.927 ± 0.93 |
| End of 24 weeks | 7.607 ± 0.97* | 7.409 ± 0.99* |
| Change in IL 6 | 0.420 ± 0.38 (5.23%) | 0.518 ± 0.29 (6.43%) |
| TNF-α (pg/ml) | ||
| Baseline | 15.728 ± 1.92 | 15.348 ± 1.69 |
| End of 24 weeks | 15.178 ± 1.85* | 14.597 ± 1.65* |
| Change in TNF-α | 0.550 ± 0.42 (3.48%) | 0.751 ± 0.42 (4.88%) |
| Adiponectin (µg/ml) | ||
| Baseline | 4.441 ± 0.84 | 4.673 ± 0.93 |
| End of 24 weeks | 5.069 ± 1.32* | 9.449 ± 1.44* |
| Change in adiponectin | 0.629 ± 0.87 (14.16%) | 4.776 ± 1.68@ (102%) |
Values are expressed as Mean ± SD. N: Number of Patient, SD: Standard Deviation Treatment A: Conventional treatment and Treatment B: Add on teneligliptin with conventional treatment.
* p<0.05 from baseline to end of 24 weeks by using paired t test (within group comparison).
$p<0.05 indicate change in Glycaemic parameter (HbA1c, FBG, and PPBG) from the baseline to 24 weeks.
#p<0.05 indicate change in lipid parameters (TC, TG, and LDL) from the baseline to 24 weeks;
@p<0.05 indicate change in adiponectin level from the baseline to 24 weeks; between groups comparison was done using un-paired t test.
Lipid parameters: Lipid parameters were found comparable in both the treatment groups at the baseline. However, there were gradual reduction in Total cholesterol (TC), Triglyceride (TG), atherogenic index (TC/HDL ratio) and Low density lipoprotein (LDL) over the period of 24 weeks in both the treatment groups (paired student t-test). The improvement in lipid profile was significant in treatment B as compared to treatment A at the end of 24 weeks. Mean changes of these parameters have been shown in Table 2.
Total cholesterol levels: There was significant decrease in serum TC levels after 24 weeks from baseline in both treatment groups. The treatment A reduced the serum concentration of TC from 218.08 ± 20.15 mg/dL to 176.78 ±13.22 mg/dL and in the treatment B 219.00 ±14.18 mg/dL to 166.90 ±19.56 mg/dL. Further, between groups comparison showed significant reduction in TC in treatment B as compared to treatment A (Figure 3).
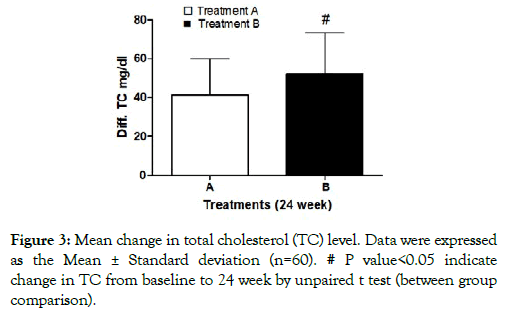
Figure 3: Mean change in total cholesterol (TC) level. Data were expressed as the Mean ± Standard deviation (n=60). # P value<0.05 indicate change in TC from baseline to 24 week by unpaired t test (between group comparison).
Serum triglyceride levels: Reduction in mean change of TG level was 30.75 ±11.56 mg/dL and 39.48 ± 15.25 mg/dL in treatment A & B. The mean reduction change (from baseline to end of study) in TG levels significantly increased in treatment B as compared to treatment A at the end of 24 week. Between groups comparison showed significant reduction in TG in treatment B as compared to treatment A (Figure 4).
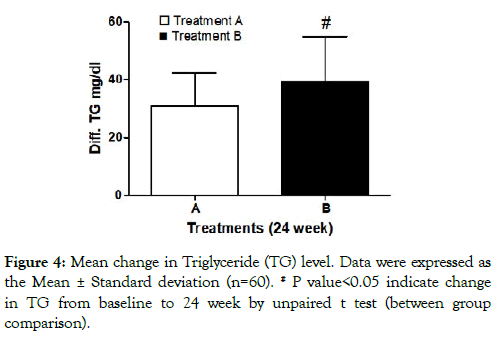
Figure 4: Mean change in Triglyceride (TG) level. Data were expressed as the Mean ± Standard deviation (n=60). # P value<0.05 indicate change in TG from baseline to 24 week by unpaired t test (between group comparison).
Serum HDL cholesterol: At the end of 24 weeks, add on teneligliptin group (treatment B) showed tendency to increase HDL level. Improvement in mean change of HDL in treatment A was 7.24% and 8.68% in treatment B.
Serum LDL cholesterol levels: At the end of 24 weeks, add on teneligliptin group (treatment B) showed tendency to reduce in serum LDL levels. Reduction in mean change of LDL in treatment B was 34.06% as compared to 28.24% mg/dL in treatment A. The mean reduction change (from baseline to end of study) in LDL levels significantly increased in treatment B as compared to treatment A at the end of 24 week. Between groups comparison showed significant reduction in LDL in treatment B as compared to treatment A.
Cytokines levels
Serum Adiponectin levels: At the end of 24 weeks, add on teneligliptin group (treatment B) showed tendency to increase adiponectin level. Mean change of adiponectin in treatment B was 4.776 ± 1.68 μg/ml as compared to 0.629 ± 0.87 μg/ml in treatment A. The mean change in adiponectin levels significantly improved in treatment B as compared to treatment A at the end of 24 week. Between groups comparison showed better improvement in adiponectin levels in treatment B as compared to treatment A (Figure 5).
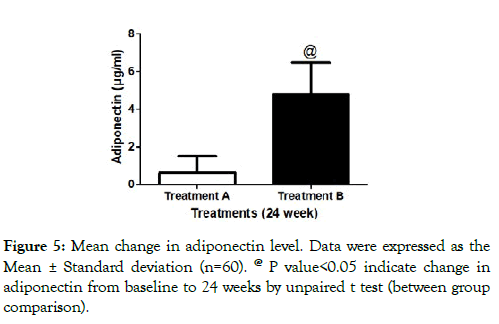
Figure 5: Mean change in adiponectin level. Data were expressed as the Mean ± Standard deviation (n=60). @ P value<0.05 indicate change in adiponectin from baseline to 24 weeks by unpaired t test (between group comparison).
In addition to above, reduction in inflammatory cytokine levels (IL 6 and TNF-α &) was also observed in treatment B as compared to treatment A at the end of 24 weeks but was not found statistically significant (Table 2).
Safety assessment: The most common AEs experienced in both the treatment groups were hypoglycaemia, constipation, abdominal pain, acidity, weakness and headache. The incidence of AE was 35% in treatment A and 21.66% in treatment B group (Table 3).
Table 3: Summary of adverse events.
| Adverse event (AE) | Treatment A N=60 (%) | Treatment B N=60 (%) | Naranjo score | Scale |
|---|---|---|---|---|
| Hypoglycemia | 2 (3.33%) | 2 (3.33%) | 9 | Definite |
| Constipation | 5 (8.33%) | 2 (3.33%) | 9 | Definite |
| Abdominal Pain | 6 (10.00%) | 3 (5.00%) | 5 | Probable |
| Acidity | 4 (6.66%) | 3 (5.00%) | 5 | Probable |
| Weakness | 2 (3.33%) | 2 (3.33%) | 4 | Possible |
| Headache | 2 (3.33%) | 1 (1.66%) | 0 | Doubtful |
| Total | 21 (35%) | 13 (21.66%) |
Treatment A: Conventional treatment and Treatment B: Add on teneligliptin with conventional treatment.
Better tolerability and safety of teneligliptin under the add on therapy.
Diabetes is progressive disease which include both microvascular and macrovascular complications [15]. The relation between DM and lipid profile has been established during the past decades [16]. Both lipid profile and diabetes are shown to be the important predictors for metabolic disturbances including dyslipidaemia, hypertension, and cardiovascular diseases [4]. Diabetic dyslipidemia accounts for around 80 percent diabetic deaths due to cardiovascular complications [17]. Diabetes is typically managed employing a stepwise approach involving diet and lifestyle modification followed by addition of oral as well as injectable antidiabetic drugs [18]. Although international and native guideline recommended lifestyle management as the mainstay of treatment for T2DM, with metformin as the preferred initial oral antihyperglycemic agent in most of the patients, but there is need for extra additional approach with synergistic effect [19].
In the present study, a trial has been made to evaluate the efficacy and safety of newly developed DPP-4 inhibitor, teneligliptin 20 mg in T2DM patients having dyslipidemia who are not adequately controlled by ongoing conventional therapy. The potential effect of teneligliptin in the management of hyperlipidemia and obesity has been established in preclinical studies [20,21]. Dipeptidyl peptidase 4 inhibitors which inhibit the endogenous glucagon like peptide 1 (GLP) metabolism and thereby increases GLP-1 level in the physiological range [22]. They act by regulating insulin and glucagon secretion [23]. Rise in new beta-cells and inhibition of their apoptosis is seen with DPP-4 which might be potentiallys improve the disease pathogenesis [24]. Teneligliptin suppresses proinflammatory activation of macrophages and adipocytes [25]. Therefore, it is a possible target for cardio protective effect.
Present work clearly demonstrated that teneligliptin addition to glimepiride/metformin stable dose significantly reduced HbA1c level as compared to conventional therapy at 24 weeks from the baseline. Further, we also observed significant reduction in blood glucose levels (FBG and PPBG) in both the treatment groups. Results of the present study are found consistent with the previous clinical reports from japan, where efficacy of teneligliptin as add on treatment decreased HbA1c, FBG and PPBG levels at 12 weeks and the same study was expanded to 52 weeks [26]. Our study suggested that, the addition of teneligliptin 20 mg to conventional therapy (metformin/glimepiride) significantly improved the efficacy of conventional therapy. Our study results support the previous clinical study wherein, combination of teneligliptin with that of insulin reduced HbA1c level and showed synergistic effect [14]. HbA1c levels in blood are one of key marker to know glycemic control [27]. In our study, tenligliptin as add on treatment significantly decreased HbA1c in patients with T2DM which might be possibly due to its synergistic action.
DM is often related to with alterations in lipid metabolism and abnormalities in serum lipid profile. Dyslipidemia is common in T2DM patients with poorly controlled glycemia [28]. We observed significant decrease in HbA1c and lipid profile at the end of 24 weeks in teneligliptin treated patients. Addition of teneligliptin showed significant reduction in TC at the end of 24 weeks. These findings are supported by one in all the meta-analysis report where in lipid lowering effect of DPP-4 inhibitors revealed a decrease in TC [29].
Beneficial effects of metformin on lipids could be because of inhibition of fatty acids released from adipose tissues, its direct effect on VLDL-C metabolism and/or secondary to enhance insulin sensitivity [30]. Elevated TG levels have been recognized as a risk factor for progression of CVD [31]. In this study, addition of teneliglptin reduced TG levels by 20.02% at the end of 24 week. Reduction in TG rich lipoprotein may include enhanced expression of the LDL receptors, increased expression of lipoprotein lipase, and reduced expression of apo C-III and very low-density lipoproteins [32]. Thus, teneligliptin might act through either of these mechanism to reduce this.
Besides, add on therapy of teneligliptin showed reduction in LDL-C levels (34.06 %) in treatment B. Meta-analysis report by Wulffle et al., showed efficacy of metformin in reducing TC, TG and LDL-C in their study with a minimum of 6 weeks of treatment in T2DM patients [33]. So, we conclude that add on therapy of teneligliptin together with metformin might give better reduction in serum LDL levels. Further, mean atherogenic index (TC/HDL ratio) was found comparable from baseline to week 24 for both the treatment groups. The mean atherogenic index decreased (30.03%) in treatment B when put next with the treatment A (28.24%) from baseline data. There is a report of significant decrease in TC, TG and LDL-C with increase in levels of HDL-C after 3 months of treatment with metformin in T2DM patients [34]. Pravin Kumar and Gokul reported a decrease of 16%, 12% and 10% of TC, TG and LDL-C respectively and a 15% increase of HDL-C; and achieving the lipid control goals with metformin - glimepiride combination therapy of 26 weeks in T2DM in their study [35].
Thus, our results were also parallel with the findings of various reports. Therefore, it will be suggested that teneligliptin addition with conventional antidiabetic therapy can be useful in controlling diabetes with dyslipidemia. Thus our results shown promising data of teneligliptin in T2DM patients with dyslipidemia.
Tumor necrosis factor-α and IL-6 are important mediators of inflammation and will provide a possible link between visceral fat and systemic inflammation. They are both known to promote lipolysis and therefore the secretion of free fatty acids, which contribute to a rise in hepatic glucose output and IR, impair adipocyte differentiation, and promote inflammation [36]. Experimental studies and cross-sectional analysis have shown that circulating IL-6 is related to hyperglycemia and insulin resistance. It’s also been shown that circulating IL-6 increases with the degree of insulin resistance [37]. The protective effects of adiponectin in the prevention of progression of insulin resistance and in cardiovascular events, and its potent influence in components of the metabolic syndrome, have made it a highly promising therapeutic target [38]. These markers though well understood in terms of their regulation in diabetes population are still lacking acceptance as clinical markers because of the variation of levels among various ethnic groups [39]. Currently limited information is available on inflammatory cytokines in type 2 diabetes and people with impaired glucose and lipid metabolism in Indian population. Our study has demonstrated significantly raised adiponectin levels in treatment B as compared to treatment A. also, an improvement in adiponectin level found associated with reduction of inflammatory cytokines (IL-6 & TNF-α) which was clearly demonstrated in our study.
Safety assessment: Clinical symptomatic assessment was done for AEs like hypoglycemia and constipation, which were considered as definite; abdominal pain, and acidity were considered as probable; weakness and headache considered as possible and doubtful respectively by Naranjo AE assessment scale. It is also to be noted that, the incidence of hypoglycemic symptoms was similar 3.33% in both the groups. For selection of antihyperglycemic agents for add on therapy it includes, individual patient characteristic, glucoselowering efficacy, risk of hypoglycemia, body weight gain, and cardiovascular benefits associated with the drugs are preferentially considered [40].
In summary, results of our study showed that, add on therapy of teneligliptin with standard therapy of anti-diabetic class of drug significantly improved lipid levels by increasing level of good cholesterol as compared to the conventional therapy, this was possibly due to synergistic action of teneligliptin. Hence, during this scenario, the present study supports the initiation of treatment of T2DM with dyslipidemia using teneligliptin, which is affordable and effective in decreasing the glycaemic as well as lipid levels together with lifestyle modifications. Further add on therapy should be initiated if treatment is not satisfactory because optimum glycaemic level is must for the reduction of elevated lipid levels and thereby preventing atherosclerosis and its complications.
In our study teneligliptin, as add on therapy was found well tolerated and effective in T2DM with dyslipidemic patient population. Tenligliptin add on treatment with atorvastatin was found better option alongwith conventional-therapy in reducing glycemic and lipid parameters as well as improvement in adiponectin levels in diabetic patients with dyslipidemia.
The authors acknowledge the guidance of Dr.Parag Shah, Dr. Krishna Shah, Dr. Alka Makad, Dr. Chirag Vaghela, Dr. Premal Thakor and Dr. Shubha Desai without their support this work would not have been possible. We also thankful to Krishna shah for providing scientific evaluation and assessment of cytokines. We are also thankful to staff of research department Jivraj Mehta Hospital and Bakeri medical research centre, Ahmedabad for extending required help and research facility for the present study.
The authors declare that they have no conflict of interest.
The corresponding author has designed the work, data collection, data analysis, statistical analysis, and prepared manuscript. Both authors discussed and provide critical feedback on manuscript.
Citation: Vinendra MP, Sunita SG (2020) Efficacy and Safety of Teneligliptin as Add on Therapy in Indian Type 2 Diabetes Mellitus Patients having Dyslipidemia. J Diabetes Metab. 10:844. doi: 10.35248/2155-6156.20.11.844
Received: 02-Apr-2020 Published: 30-Apr-2020, DOI: 10.35248/2155-6156.20.11.844
Copyright: © 2020 Vinendra MP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.