Research Article - (2021) Volume 12, Issue 10
Our aim was to check the suitability of the human intestine for the delivery of the re-combinant proteins in humans. The prospective applications of said technology are huge, since travel in the outer Space to locate new planets for the relocation of the overcrowded Earth will face the need for the crew vaccination from the pathological microorganisms, found in these outer Space locations. We invented the technology to treat Diabetes II and his chronic anemia in the adult volunteer as the draft of said new applications of said in vivo technology. The strain of lactobacilli from normal human intestinal micro-flora was isolated, genetically engineered and then returned back for the adhesion back to the host intestinal epithelium of the volunteer. The volunteer was cured from his Diabetes II and chronic anemia. We discuss the potential applications of said technology for the in vivo expression of the vaccines for the crews of the outer space long range travel missions coming soon.
Normal intestinal micro flora, Intestinal lactobacilli, Lactobacillus casei, Recombinant human insulin, Recombinant human erythropoietin, Long range outer Space flights, Manned crew vaccinations
An adult individual, working for our businesses as the electronics engineer, has approached us, when the work has been done, and kindly asked to help him. He is in his 50-s. During his years before 30 yo, he suffered acute pielonephrits after immercion of his body to a very cold environment. He developed acute pielonephritis at that age, which was not completely cured and became chronic pielonephritis, com-plicated with the chronic anemia said individual has developed due to the absence of the erythropoietin production by his damaged kidneys. Said individual has developed anemia and Diabetes II with his aging, daily taking metformin and three times a year requiring hospitalization for the blood transfusions and the blood formula checking after that. With the time, he became resistant to the metformin taken and after his visit to the doctor has received the prescription to add insulin injections to his daily routine along with still taking the metformin. The erythropoietin deficiency stayed constant, complicating the life of said individual. He was very much upset, since now he had to monitor his blood glucose level multiple times over the day, adjusting the dose of the insulin, while having the administered insulin and metformin still taken after he had become resistant to it. So, he approached us and said, that he already saw multiple genetically engineered biocatalysts, that our businesses have created, and he stated, he believes in us and asked us to cure him from his Diabetes II and help him with his anemia, using the new recombinant engineered strain, he wanted us to construct for him. We said him that he would be the volunteer, and we will help him. Our decision was based on our knowledge, that the volunteer most likely has Lactobacillus case in his intestine, and we can make this organism expressing recombinant human insulin and recombinant human erythropoietin in situ.
Latobacillus casei was originally isolated from the intestinal tract of the infant [8. Lactobacillus species, one the main components of the human gut microbiome, are the part of many probiotic supplements, widely used to treat gastrointestinal and other ailments, associated with the anti-inflammatory and immune-modulating ef-fects, that are generally thought to promote health, but are not understood in detail [1,3,7,8,10]. L. casei is the one of the dominant species in the gut of breast-fed in-fants [1,2,6,8,10]. Said commercial probiotics with lactobacilli do not adhere to the intestinal epithelium after intake, and their action is only connected to their constant intake in large amounts [8, 10]. Due to strong beta-gactosidase activity in strains of L. casei L. casei is capable of growing on various types of milk [8,10].
Therefore, we have decided to use the intestinal Lactobacillus casei of the volunteer to achieve the intestinal expression of the human insulin and human erythropoetin in vivo (this report). Specifically, our choice came to isolation of L. casei, as it was shown before, it is a widely used as the probiotic strain added to the regular meals [1,4,8,10].
The details of the procedure to cure Diabetes II and the anemia of the adult individual and their future prospects and developments are the objective of this manuscript. The estimated national cost of diabetes in 2017 was $327 billion [25]. More than 34 million Americans have Diabetes (about 1 in 10), and approximately 90- 95% of them have type II Diabetes [25]. Therefore, the evidence of the cost of our way of treating Diabetes II and this certain type of the anemia is obvious. Herein we have postulated for the first time the principles of how to make the ingested probiotic strain adhere back to the host intestinal epithelium, therefore, its action is permanent, until the individual makes something to inhibit and eliminate said probiotic strain from the individual’s intestine, like the intensive antibiotic therapy, and/or the alcohol abuse. After that, the procedure of the isolation, genetic engineering for the adhesion back to the host intestinal epithelium has to be performed again. Therefore, the estimated national cost of the future intestinal re-combinant proteins availability proposed herein has to be evaluated upon the availability of said data.
Isolation of the Lactobacillus casei, the component of normal intestinal microflora, suitable for the construction of the insulin-producing and erythropoetin producing strain to be returned back to the patient’s gut to cure the patient from Diabetes II and erythropoetin insufficiency.
We have developed the selective medium to isolate lactobacilli from the intestine of the volunteer. Lactobacilli are the naturally occurring intestinal microorganisms, obligate microaerophiles, which are transferred from the mother's intestine to the mouth of the newly born child during the natural birth process. If the birth was conducted via the C-section, then the ingestion of the mother’s lactobacilli does not happen, and said lactobacilli may be ingested by the newly born child from the prepared in advance broth culture of the mother’s lactobacilli with all the necessary controls of said procedure performed as needed. Mother's Lactobacilli protect the newly borns from a variety of potential food-borne pathogens up to one year of the newly born's life [5]. Lactobacilli produce organic acids, and hydrogen peroxide along with the potentially beneficial naturally occurring antimicrobial proteins in the host intestine. The most important, the produced by lactobacilli acetic acid has the strong antibacterial activity [8]. The adhesion back to the intestinal epithelium happens only in a very short time for the isolated lactobacillus culture to be maintained outside of the intestinal content of the host organism. Therefore, to make sure, that the isolated lactobacilli will again adhere back to the intestine of the volunteer, we made sure, that the isolation of the intestinal lactobacilli will be on the special selective medium to ensure, that it would take only 48 hours to grow up the intestinal lactobacilli of the volunteer and, therefore, only 192 hours to get the recombinant L. casei capable of adhesion back to the volunteer's intestine. Any elongation of said time of being outside of the intestinal content of the host leads to the irreversible loss of the capability to adhere back to the host intestinal epithelium [8]. As we have described herein, said recombinant L. casei would be capable of expressing the recombinant insulin and the recombinant erythorpoetin at the gut of the patient, our volunteer, specifically. Therefore, the total cell duplication time did not exceed 120 cell duplications (lactobacilli cell duplication time under the chosen conditions of growth is 1 h), as it is mandatory to store intact the capability to adhere back to the intestine of the host [8,10,30].
Therefore, we have used the constructed by us earlier described the Selective Me-dium to Isolate Intestinal Lactobacilli from the feces of the volunteer [8,10]. Herein we describe the adjusted to our conditions the selective medium for isolation of lac-tobacilli, g/l:.
Casein Tryptone 10,0;
Meat Extract 10,0,
Yeast Extract 5,0, K2
HPO4 2.0,
NaH2PO4 2.0,
Tris 2.0,
MnSO4 0.8,
X-gal 0.4,
Na Acetate 20.0,
Ox Gal 6.0,
Amphotericin powder (added after autoclaving and cooling down to 45oC) 0.4,
Distilled water to 1 L,
after adjusting the medium pH to 5.9.
The significance of the nutrient and selective components of the Selective Lactoba-cillus Medium [10]: The rich Selective Lactobacillus Medium composition, presented by Caseine Triptone, Meat Extract and Yeast Extract ensures that intestinal lactoba-cilli grow on the selective medium for 48 hours aerobically at 37°C. X-gal (5-Bromo-4-Chloro-3-Indolyl β-D-Galactopyranoside) is the selective agent, showing the presence of beta-galactosidase activity in L. casei isolates [21].Sodium acetate in the Selective Lactobacillus Medium is the inhibiting agent for many intestinal microorganisms, except intestinal lactobacilli. X-gal in the Selective Lactobacillus Medium will ensure, that the colonies of L. casei will be dark blue, when they grow on the Selective Lactobacills Medium as described [10]. Ox gal is the selective agent, originating from bovine bile, serving as the bile substitute in the Selective Medium to isolate intestinal lactobacilli and works as the selective agent to inhibit growth of enterobacteria and some other intestinal microorganisms [8]. Amphotericin B is the anti fungal agent [11]. It is added to the Selective Lactobacillus Medium to inhibit growth of molds and candida, should they happen to be in the feces, used to isolate intestinal lactobacilli [8,10].
The Selective Lactobacillus Medium without amphotericin B was autoclaved at 0.5 atm for 30 min. The selective agent Amphotericin B powder was added to the Selective Lactobacillus Medium after autoclaving of the medium, when it was cooled down to 45°C for the distribution into Petri Dishes [23]. Selective Lactobacillus Medium was cooled down to 45°C and the amphotericin powder was added. The medium was poured by 20-25 ml in the 100 mm Petri dishes, which were dried in the Laminar Flow Cabinet [15].
Dried medium in the Petri dishes was marked with the permanent marker [21], making five sectors of the approximately equal size. The 20 microliter aliquotes of the decimal 3rd, 4th, 5th, 6th and 7th dilutions of the volunteer faeces in the dilution buffer (DB) were plated on each Petry dish by P200 pipetman [19]. Each pipetman aliquote was then spreaded on the whole surface of the medium sector (not to cross the neighboring sector), using the plastic microbiological loop [16]. The plates then were incubated in the thermostate at 37°C for 48 hours.Colonies from the last feces dilutions were stricked once on the Selective Lactobacillus Medium to make sure the pure cultures were isolated. Purified lactobacilli strains were stored at -80°C, each in 300 microliters of the liquid Selective Lactobacillus Medium, added to the sterile 1.5 ml Eppendorf tubes [22] before the inoculations.
The DB composition: 0.1 M Tris, 0.15 M NaCl, 1.0 M urea, 10 mM CaCl2, 0.1 M citric acid monohydrate, 5 g BSA and 1.0 g cysteine-HCL (pH 6.00). The DB was sterilized by autoclaving at 1 atm for 30 min. The DB was distributed into the sterile 1.5 ml Eppendorf tubes by 900 microliters.
Dilution of the volunteer feces: The 0.1 g of the freshly collected volunteer's feces was dissolved in the first 1.5 ml Endorphin tube, containing 900 micro liters of DB (the 1st dilution of the feces). The O.1 ml of the resulting the first dilution of the volunteer feces were transferred to the second Eppendorf tube with 900 microliters of DB (the 2nd dilution of feces), and so on, to make the necessary for the plating decimal dilutions of the volunteer's feces. The procedure to dilute feces was, as follows. The 100 microliter aliquiotes of the first dilution of the volunteer feces were added [19] to the second 1.5 ml Eppendorf tube with 900 microliters of DB, etc, to make the second, the 3rd, 4th, 5th, 6th and 7th, etc. dilutions of the feces of the vol-unteer.
It is very important, that the isolated strains of lactobacilli were isolated and purified in 96 hours, then stored at -80°C in the same medium, as used for the isolation and purification of the intestinal lactobacilli, only without agar-agar. The time to obtain the recombinant strain of L. casei should not exceed another 96 hours. Therefore, the total time, which the isolated intestinal lactobacilli were outside of the volunteer's intestine, did not exceed 192 hours. As that was stated before [8,10], only in such case the ingested recombinant strain of Lactobacillus casei, which was maintained outside of the volunteer's intestine, regains the capability to adhere back to the host intestinal epithelium and therefore, is capable to express the sub cloned recombinant human insulin and recombinat human erythropoetin in vivo (this report). The total time to isolate, purify and metabolically engineer L. casei took only about 192 hours. As stated before [8], any elongation of the time for being outside the volunteer's intestine removes the capability of the isolated culture to adhere back to the volunteer’s organism intestinal epithelium, and, therefore, makes the attempts to get the constant recombinant human insulin and recombinant human erythropoetin expression in vivo futile (this report).
Construction of the recombinant Lactobacillus casei, excreting recombinant human insulin and recombinat human erythropoietin:
We have used Lactobacillus casei MT896 strain, isolated from the volunteers intestine, to do the metabolic engineering and to make sure, that said strain would be capable of expressing and excreting of the human recombinant insulin, when that would be returned back to the host intestine. Using genome tailoring methodology [12], in Lactobacillus casei MT896 strain we have removed the unnecessary for the strain maintenance genes at their positions 156316...156498 bp, 156587...156787 bp, 156896 ...157264 bp, 160931...161227 bp, 168874...169860 bp, 178189...179010 bp, 187491...188624 bp, 201318...203045 bp, 214779...215498 bp, 216403...217710 bp, 220032...220799 bp, 293172...295181 bp, 301768...302604 bp, 324591...325823 bp, 375014...377602 bp, 377602...378726, 424955...425782, 471463...473505, 476681...477658, 530517... 531566, 578066...580270, 623394...624161, 642356...643819, 651843...652904, and 684866...685807 bp [11,12,18, 26, 31]. The recombinant strain of Lactobacillus casei was produced as described [12,26]. The sequence of the recombinant human insulin and the recombinant human eryth-ropoetin, expressed by the intestinal isolate Lactobacillus casei MT896InsulinErythropoetin, has been deposited to NCBI (Submission ID is 2442803).The sequence of the recombinant human erythropoetin expressed by the intestinal isolate L. casei MT896InsulinErythropoetin has been deposited to NCBI (Submission ID is 2482572).
The recombinant human insulin producing strain of Lactobacillus casei, Lactobacillus casei MT896InsulinErytropoetin, was then developed as described [12,26].
The recombinant human insulin and the recombinant human erythropoetin producing strain of Lactobacillus casei MT896InsulinErythropoetin was created [12,26]. The primers for the PCR for the recombinant human insulin to check out the presence of the recombinant human insulin gene in the recombinant strain of intestinal lactobacilli Lactobacillus casei MT896InsulinErythropoetin were designed, using the publically available tool at the NCIB web site [20]. The PCR with the recombinant insulin primers (the forward primer AGCATCTGCTCCCTCTACCA and the reverse primer TCCATCTCTCTCGGTGCAGA) showed the production of the human recombinant insulin by Lactobacillus casei MT896InsulinErythropoetin, as that was confirmed clinically later on in this project.
The primers for the PCR for the recombinat human erythropoetin gene in the recombinant strain of intestinal lactobacilli Lactobacillus casei MT896InsulinErythropoetin were designed, using the publically available tool at the NCIB web site [20]. The PCR with the recombinant human erythropoetin primers (the forward primer CACGAATGTCCTGCCCTTTG and the reverse primer TACTTCAGCTTTCCCCGGAG, showed the production of the human recombinant erythropoetin by Lactobacillus casei MT896InsulinErythropoetin, as that was confirmed clinically later on in this project.
The Clinical effect of ingestion of the Lactobacillus casei MT896InsulinErythropoetin by the volunteer:
Shortly after ingestion of the 25 ml of the Selective Lactobacillus Medium, containing frozen stock of Lactobacillus casei MT896InsulinErythropoetin, the volunteer has achieved its adhesion to the intestinal content, as he noted the absence of his Diabetes II clinical symptoms after the regular meals, taken three hours after the Lactobacillus casei MT896 Insulin Erythropoetin intake. Said volunteer also did not need any insulin injections after his meals, as we monitored his blood glucose concentrations one hour after said meals (this project). There was no glucose in the urine of the volunteer (tested in our lab), and the blood glucose levels exceeded 100 mMols/d only one hour after the meals, or corresponded to the normal blood glucose levels of the normal individual. His symptoms of the anemia have been also gone. With our sincere interest, we have ordered the volunteer, who has continued to report for the regular work, to do the three days continuous blood glucose testing, especially one hour after having meals with even the sugar content. The data, obtained during said testing, have convinced us, that there was no the need for the blood glucose monitoring daily, only once a week, since the volunteer seemed to be free from Diabetes II clinical symptoms.
No any signs of the volunteer’s anemia were observed, and he was advised to go to the clinic and have his blood formula checked. The volunteer was advised to repeat his periferial blood formula testing in the next three weeks interval. Three weeks passed. No blood glucose level spikes above 118 mMols/d were ever observed, even after sugar-containing meals. The conditions of maintenance of Lactobacillus casei MT896InsulinErythropoetin in the volunteer’s intestine were carefully discussed with the volunteer to make sure that under no circumstances he regains again his existing Diabetes II and his existing anemia clinical manifestations and he continues to work for us to be monitored for the blood glucose sugar. So, his work was linked to his job more and he felt much more satisfied and confident compared to the time before this treatment.
We have published before our experience of the in situ availability of the human recombinant insulin expressed by genetically engineered by us recombinant strain of Bifidobacterium breve bb387Insulin [30]. In this report we have described the effects on the blood glucose metabolism maintenance and the coping with the existing chronic anemia of the volunteer.
As that was shown at [29] and as that was discussed herein per the prospects of our planet in the future, we have noted the coming in the future years from now the shortage of the fresh water coming in the next 20 years or about that (we are not mediums to make said predictions). Indeed, accumulated in the air CO2 is one of the heaviest gasses in the air blend, reaching its density 1.997 g/ cubic meter [11,29]. The CO2 in the air gas mixture under no wind conditions spreads on the ground surface and selectively absorbs all the infrared energy of the Sun, thus heating the ground significantly, up to the water boiling point in Texas and some other Southern states of the US. That causes the extra evaporation of the fresh water from soil to the air as shown in Figure 1.
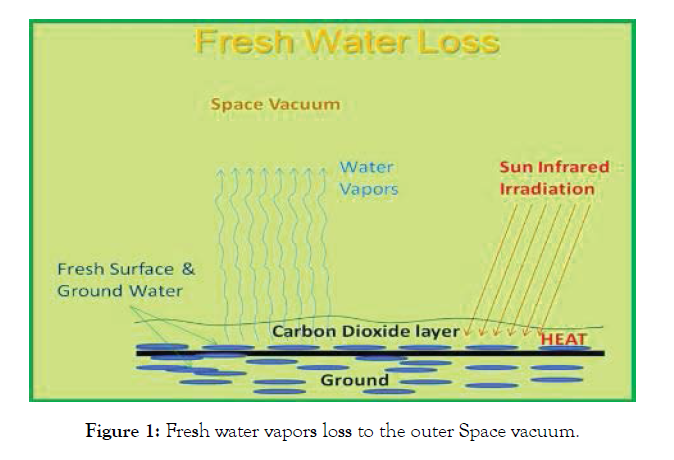
Figure 1: Fresh water vapors loss to the outer Space vacuum.
As you know, Global Warming presents itself in various forms, specifically with increased frequency of tornadoes, rains, etc. But the Earth gravity has been stable for the last few million years from now. Therefore, under the constant gravity force applied, more fresh water vapors are in the air. The Space, surround-ing Earth, as any Space anywhere, has deep vacuum. That vacuum sucks fresh water vapors from Earth air right from the Earth’s atmosphere, and such fresh water vapors travel in the Space in the unknown direction away from our planet. In 2010 NASA has bombarded the Moon and found plenty of ice on its dark and very cold surface. They were guessing, where said ice came from? Located 220000 miles apart from the Earth Moon worked as the cold trap for the fresh water vapors coming from Earth in the Space vacuum [29]. What will happen next and, the most important, when? It is impossible to anticipate, that the fresh water loss to the outer Space may be stopped at any time even if the Earth population is suddenly decreased in its amount. The extra air CO2 comes from the intensified petroleum use, and from the use of products of the petroleum distillation at said refineries (gasoline, diesel fuel, etc.) for combustion, producing CO2. People breath and produce CO2 as well. It is anticipated 14 billion people on Earth by 2050 [29]. That increases more the air CO2 content, leading to the accelerated fresh water loss as discussed. We have no any idea, what will happen soon, if no new planets, similar to Earth, will be discovered and the overcrowded Earth population will not start to move there. We do anticipate, that by that time a glass of water will be available for significant money, and there would not be no washing of our bodies and clothes, no the crops and the livestock production. No the livestock and the crops production are anticipated with the reasonable justification above. The solution to save our planet’s fresh water would be to restructure the current economy for inclusion as wide as possible of Dr. Tyurin’s private technologies of the manufacture of the commodity chemicals and fuels, made now solely from air CO2, N2 and the hydrogen [11,29], projected to be produced by the electrolysis of the petroleum production waters and other heavily contaminated waters, while the byproduct oxygen adds to the air, and the proposed hydrogen would be produced at $0.20 per 1 kg or 500 moles [29], and the electric energy for said distillation of the sea water is obtained for free at located at the Equator surrounding latidtudes.
The prospects of the future use of this recombinant technology and its potential ap-plications are discussed herein as related to the coming soon long range outer space flights of the manned crews, hoping to find the appropriate place for humans to reside in the Universe due to the enormously increasing Earth population, doubling every 35 years, and going to reach 14 billion people by 2050 [29]. Private in-vestors started investing much into the coming soon prospective long range outer space missions of the manned crews[27]. The US model of the economic develop-ment has been proven to be immaculate and directed only to moving forward. We are here, in the US, we are ready to do anything possible to make the life of said manned crews as simple as possible [28]. We offer 1) the crew vaccination in situ via the described herein way of Diabetes II treatment in the adults and 2) foods for the manned crews, which they can prepare themselves during said long range space flights. Both our offers are intended to decrease the lift-off weight of the spacecrafts. The meals, that we offer to use, are in the next our possible manuscript in this magnificent peer-reviewed Journal. The procedure of making the recombinant strain(s) from the intestinal content is carefully described herein, as that pertains to the expression in vivo of the recombinant protein, the recombinant human insulin and the recombinant human erythropoetin.
Imagine, that the long term outer space mission has reached something which they consider to further explore as the potential damger for the proposed relocation of the crowded Earth population to the found in the outer space planet. Imagine, that the samplers have taken the biological samples to be detail examined for the potential microbial dangers for the crew of said long range spacecraft. Certain objects may be determined as causing damage to the crew, which has never been in contact with said potentially dangerous organisms in the outer Space location discovered. Now, we offered herein the technology to produce the recombinant therapeutic proteins right in the intestine of the particular individual chosen. In general, the expression of any new recombinant antibody, new vaccine and any other forms of the therapeutic recombinant proteins will work. The technology used, metabolic engineering, as described by Dr. Tyurin, does not have any size of the cloned DNA limitations (in the reasonable context, of course). It is extremely important, that the intestine, where the recombinant proteins are produced, is in the tight connection with the whole human organism and, therefore, will ensure the random blood distribution of said recombinant protein(s). Therefore, the intestine will work as the internal gate for the therapeutic proteins. As such, said manned crew members may get the essential vaccines in their own bodies in just less than 200 hours. This approach may have crucial importance for the manned crews life during said long term outer space missions proposed. This is the first ever report on the use of the recombinant strain, producing recombinant proteins and returned back to the host intestine, capable of adhesion back to the host’s intestinal epithelium. The technology of said recombinant strain production is associated with the genome tailoring technology discovered ad described in detail by Dr. Tyurin [12], for which there are no limits on the size of the inserted into the genome of the recombinant strain the recombinant DNA. Therefore, we offer for the first time ever 1) the existing and well known gate for the delivery into the human blood stream of the recombinant proteins and 2) no any limitations on the size of said recombination proteins, making this technology the technology of choice to construct the intestinal human bacteria, expressing vaccine proteins back in the human body. This makes the value of our technology much more, than it is only for the treatment of the Diabetes II ($327 billion for the US only in 2017).
The Author had no special funding.
The Author’s corporate web site [29] was brutally destroyed by the attorney from Hirsch and Westheimer law firm, who in the civil courthouse (Harris County, the State of TEXAS), who openly stated at the materials submitted to the Court of law their paperwork, that she kept connected to Dr. Tyurin’s corporate computer, she knew all his passwords, etc. She apparently went to the yahoo small business web site and opened access to Dr. Tyurin’s corporate web site [29], where she apparent-ly has changed his credit card number and/or its expiration date. After that the web site has died, while Dr. Tyurin became homeless and could not see that timely. The homeless Author was gun robbed in Houston, TX, loosing all his corporate cash $10,000, credit cards and the corporate computer to the armed thief shortly after Dr. Tyurin’s visit to the major petroleum and gasoline/diesel fuel producing company in Houston, TX, to which the Author offered his proprietary technology of gasoline manufacturing from air CO2, not from petroleum, with the manufacturing cost of $ 0.35 per gasoline gallon [12, 29]. The major petroleum gasoline/diesel fuel producing companies spend $1.70 to make 1 gallon of the gasoline from petroleum (refineries are expensive in building and maintenance). With big NO in response, shortly after that visit to the major gasoline producing company, the Author had a very strange corporate car crash on the US59 with his perfect driving record and with the subsequent neurosurgery to fix the bleeding head blood vessels - the attempted murder of the Author in Houston, TX. With the subsequent neurosurgery after said car crash, the Author was unable to find the lawyer to recover the moneys in Houston, TEXAS. The attempts to get the follow up from the Houston Police and the Houston FBI were fruitless, as that appears to the Author, they are all corrupted by the petroleum businesses in TEXAS. The Author has stopped all his business operations after that day to stay safe/alive.
FUNDING was done by the private investors, who declined to provide their names and their business affiliations. The investors noted, the author should decline any source of funding. Conflict of interests. The author declares his personal conflict of interests with the law firm in Houston, TX Hirsch and Westheimer, with the major petroleum and gasoline/diesel fuel companies in Houston (TX), with the Houston Police (City of Houston) and with the Houston FBI. Ethical Approval. This article does not contain any section, requiring Ethical Approval. Consent to participate: this article is the opening gate for the wide use of the recombinant proteins in situ. Our volunteer has made his written contest to participate in this study.
Citation: Tyurin MV (2021) Expression in Situ of the Recombinant Human Erythropoetin and Recombinant In-Sulin. J Diabetes Metab. 12:900. doi: 10.35248/2252-5211.21.12.900
Received: 15-Sep-2021 Published: 27-Oct-2021, DOI: 10.35248/2155-6156.21.12.900
Copyright: © 2021 Tyurin MV. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.