Research Article - (2020) Volume 11, Issue 10
Background: Sinigrin, an aliphatic glucosinolate, is absorbed from the intestine as allyl isothiocyanate (AITC) and conjugated with glutathione (GSH) followed by excretion as an N-acetylcysteine (AITC-NAC) into the urine. AITC is the crucial metabolized form which reflects the occurring bioavailability of dietary sinigrin. However, whether the anti-diabetic effects of dietary sinigrin and the quantitative of metabolic parameters AITC and AITC-NAC remain unknown in the type 2 diabetes model (T2D).
Methods: A total of mice were divided into 6 groups: (i) normal control (ii) diabetic control, (iii) normal + 15 (μmol sinigrin/kg BW for all), (iv) diabetes + 15, (v) normal + 30 and (vi) diabetes + 30. After oral administration of sinigrin for 21 days, plasma, tissue, and urine were collected for analysis of metabolic parameters.
Results: Administration of sinigrin reduced plasma glucose and significantly improved the insulin resistance of T2D mice. Besides, the treatment of sinigrin induced accumulates AITC levels in the liver and pancreas tissue results in enhancing GSH levels in these tissues (P<0.05). Sinigrin causes less elimination of AITC-NAC in T2D mice urine (below 10% excretion compared to 70% in normal mice P<0.05). These results indicated the collaboration of changeable sinigrin metabolism for its protective effect on T2D mice.
Conclusions: Dietary intake of sinigrin possesses anti-diabetes and the changeable amount exposes AITC-NAC and AITC accumulation in the target tissue, enhancing the GSH levels may contribute to its protective effect. These findings may further justify the importance of sinigrin in phytomedicine.
Sinigrin; Type 2 diabetes; GSH, AITC; Bio-marker AITC-NAC
Increasing the consumption of vegetables, rich in polyphenols, is associated with preventing major chronic diseases, as indicated by epidemiological studies [1,2]. Glucosinolates (GLSs) are a diverse group of phytochemicals that are particularly abundant in Brassicaceae vegetables such as broccoli, cabbages, radish, and mustard that we often take. The GLSs are hydrolyzed by plant myrosinase or our enterobacterial myrosinase mainly into isothiocyanates (ITCs) [3], which are responsible for pharmacological effects [4]. Extensive research has been dedicated to evaluating the role of ITCs and GLSs in the chemoprevention of cancers [5].
Because abundant evidence of GLSs and ITCs are inhibitors of phase I enzymes and potent inducers of phase II enzymes, which is thought responsible for the removal of carcinogens [6,7]. On the other hand, evident research regarding ITCs or GLSs intake related to other physiological activity is limited. Recently, Ma et al., 2018 dedicated the clinical study which examined the relationship of intake GLSs from the source of Brassicaceae vegetables was associated with a moderately higher risk of T2D in US adult [8]. Besides, a few research has been investigated the dietary of broccoli that suppresses the oxidative stress and improves the antioxidant capacity in diabetic patient and rat [9,10]. Also, the supplement of broccoli sprout suppresses insulin resistance in T2D patients [11].
Researchers claim the role of sulforaphane, as known as abundant ITC in broccoli source, contributes to its activity in improving the risk of T2D. However, these studies performed with crude materials in a clinical study, and the actual amount of GLSs and ITCs did not quantify in the body. To elucidate the anti-diabetic activity of GLSs in vivo, it is required to analyze effective amounts of ITC after intake GLS-containing vegetables.
As shown in Figure 1, sinigrin, one of the common GLSs, is metabolized mainly into allyl isothiocyanate (AITC). Once absorbed, the primary metabolic route for AITC is conjugation with glutathione (GSH) followed by conversion via the mercapturic acid pathway to an N-acetylcysteine (NAC) conjugate which is excreted into the urine [12]. Allyl isothiocyanate (AITC) and the bio-marker AITC-NAC are the crucial metabolized forms in the metabolism pathway of sinigrin in vivo which reflect the occurring bioavailability of dietary of sinigrin. To date, there is little precise information on both on the evident anti-diabetic effects by GLSs and the distribution of GLSs metabolites in vivo. In the current study, we investigate the effects of daily intake of GLS using purified sinigrin on type 2 diabetic mice model with quantitative data of AITC in blood, tissues, and AITC-NAC in the urine. We are trying to explain the anti-diabetic effects of GLSs based on demonstrated changes in metabolic parameters in mice body.
Figure 1: Mean values of TG at three measuring times.
Chemicals and reagents
Sinigrin was obtained from TCI America, a division of the Tokyo Chemical Industry (Tokyo, Japan), the purity ≥ 98% (HPLC). Allyl isothiocyanate (AITC), phenyl isothiocyanate (PITC), L-cysteine, streptozotocin and 1,2-benzenedithiol were supplied by Wako (Wako Pure Chemical Industries, Tokyo, Japan). All reagents were purchased from Sigma-Aldrich and were used as received without further purification: N-Acetyl-L-cysteine (NAC, ≥ 99%), L-glutathione reduced (GSH, ≥ 98.0%), L-glutathione oxidized (GSSG, ≥ 98.0%), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB, ≥ 98%), ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA, 99.0-101.0%), β-nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate (NADPH, ≥ 97%), glutathione reductase from baker’s yeast (S. cerevisiae) (GR, 100-300 units/mg protein (biuret)), sodium phosphate dibasic (Na2HPO4, ≥ 99.0%), sodium phosphate monobasic dihydrate (NaH2PO4·2H2O, ≥ 99.0%), 1-chloro-2,4-dinitrobenzene (CDNB), metaphosphoric acid (MPA, ≥ 33.5%) and triethylamine (TEA, ≥ 99.5%).
Animals
Six-week-old mice Slc:ddY mice from SLC (Hamamatsu, Japan) were housed in an isolator caging system maintained at 24 ± 1°C and 50 ± 10% humidity under a 12-h light/dark cycle, and had free access to water and a standard laboratory feed (Labo MR Stock, SLC) for 1 week to accustom them to their surroundings. All experimental procedures described were approved by Guidelines for Animal Experimentation of Tokyo University of Marine Science and Technology.
Induction of Type 2 diabetes
The mice were divided into two groups as diabetes and normal group (each group n=18). Mice have fasted for 20 h before by single i.p. injection with 125 mg/kg of streptozotocin (STZ; N-nitroso derivative of glucosamine is a broad-spectrum antibiotic extracted from Streptomyces acromogenes) to induce T2D [13]. STZ freshly dissolved in 0.05 M citrate buffer, pH 4.5. Normal mice were injected with an equivalent volume of citrate buffer. An animal was accessed as being diabetes if the blood glucose level (Glc) was greater than 300 mg/dL after a 1-week injection [14].
Experimental protocols
A total of 36 male mice was partitioned into six groups (n=6 for each) and treated as follows: (i) normal control, (ii) diabetic control, (iii) normal + 15 (μmol/kg BW for all), (iv) diabetes + 15, (v) normal + 30 and (vi) diabetes + 30 as shown in Figure 2. Mice were orally administered with sinigrin (μmol/mL/kg BW) dissolved in distilled water. The effective dose of sinigrin was chosen from the consumption amount of Brassica vegetables according to a clinical trial and a pilot study. Daily consumption of GLS (3-25 mg/day/ head) in Brassica vegetables was converted to 6-12 mg (15-30 μmol)/ kg/head as a sinigrin amount. In the pilot study for 21 days, we did not detect any side effects against body weight and fundamental blood parameters in mice by oral administration of the same dose of sinigrin/kg BW/21 days (data not shown). Thus, doses of 15 and 30 μmol sinigrin/kg BW were used in the present study.
Figure 2: Trigliceride values in patients with DM and no DM at admission (T0), after the initial treatment with fluid therapy and Insulin Aspart (T1) and after folow up therapy (T2).
Mice were orally administered sinigrin solution consecutive 21 days. At the end of the 21-days, mice were killed by cervical dislocation. The blood, liver, and pancreas samples were taken, and biochemical parameters were analyzed. Bodyweight, food, and water intake were also measured in the period of the experiment.
Outcome measurement
To measure fasting blood Glc levels, blood samples were taken from the tail vein after fasting for on days -7, 0, 1, 4, 7, 10, 13, 16, 19, and 21. Insulin and triglyceride concentration were also determined on the 21-days. Blood samples were centrifuged at 9,000 g for 3 min, and plasma was obtained for the assay of the biological parameter. Plasma glucose, insulin, and triglyceride were determined using commercial reagents: Glucose CII-test Wako (Wako Pure Chemical Industries, Tokyo), ELISA Insulin kit (Morinaga Institute of Biological Science, Yokohama), Triglyceride (Wako, Tokyo), respectively. Homeostasis model assessment (HOMA) and quantitative insulin sensitivity check index (QUICKI) have been used to quantify degrees of insulin resistance and β-cell secretory capacity. The principle of HOMA is that blood glucose and insulin concentrations are related by the feedback of glucose on β-cells to increase insulin secretion. HOMA is an index of insulin resistance whose value should increase with increasing insulin resistance. QUICKI is an index for measuring insulin sensitivity (IS), which is the inverse of insulin resistance (IR). This index decreased in insulin resistance compared to normal. HOMA and QUICKI measures of insulin resistance are usually in very close agreement. HOMA =(Insulin x Glucose)/22.5 (glucose concentration in mM), QUICKI =1/[log (Insulin)+ log (Glucose)] [15].
Allyl isothiocyanate metabolite determination
Allyl isothiocyanate (AITC) levels in plasma and tissue were determined by cyclocondensation assay with slight modification [16,17]. Removal of protein in plasma and tissue homogenates with polyethylene glycol (PEG) results in preparation suitable for this assay without loss AITC. Briefly, plasma or tissue was incubated with a final concentration of PEG 6% on ice for 10 min, and then centrifuged at 10,000 g for 5 min at 4°C. Each supernatant (100 μL) was mixed with 10 mM 1,2-benzenedithiol in methanol (600 μL) and 0.1M phosphate buffer (pH 8.5, 500 μL), and incubated for 2 h at 65°C. AITC was quantitatively converted to 1,3-benzodithiole- 2-thione. After cooling and centrifugation, 2 μL was injected onto the reverse phase HPLC column (ODS 4.6 i.d. × 250 mm, 5 μm particle size) and eluted at a flow rate of 1 mL/min with an isocratic mobile phase (80:20 = methanol: water). Various concentrations of AITC solutions were used to make the calibration standard after reaction with the same reaction. To guarantee of validation and quality, phenyl isothiocyanate (PITC) was added into the blank plasma or tissue at concentration 1 nmol/mL to determine the recovery of analysis. The recovery of PITC added in the original plasma and tissue homogenate was almost completely recovered up to 98%.
Synthesis and identification of AITC-NAC and PITC-NAC as analytical standards for urine analysis
The NAC conjugate of AITC and PITC were synthesized using the published method [18] and confirmed by LC-MS. Briefly, Allyl Isothiocyanate (5.73 mmol) was dissolved in 5mL of ethanol and gradually added to a solution of NAC (5.12 mmol) and sodium bicarbonate (5.63 mmol) in 10mL of DW. All NAC has reacted within a few hours after AITC was added. Ethanol was evaporated and the mixture was acidified with HCL which gave a yellow oily precipitate. The mercapturic was extracted with ethyl acetate and traces of organic solute were evaporated using a high vacuum, yielding 4.25 mmol (83%) as a yellow sticky product. The synthetic of AITC-NAC was used to construct a standard curve to determine the concentration of AITC-NAC in the urine sample. The internal standard PITC-NAC was also synthesized as the process of AITCNAC. The product was crystallized from boiling ethyl acetatemethanol, yielding 69.8% as white crystals.
The AITC-NAC was separated by the ODS-HG-5 column (2.0 i.d. × 250 mm, 5 μm particle sizes). The mobile phase consisted of 1% formic acid in water (A) and methanol (B). An initial condition of 0% B increased linearly to 30% at 5 min, 100% B at 30 min, and equilibrated back to 0% by 52 min.
Mercapturic acid of PITC and AITC was detected as [M+H]+ in positive mode with the strong m/z = 299 and 263 as the major peak respectively, based on the previously published spectra [19]. With our analysis conditions, these synthesized samples of PITC-NAC at 26.22 min and AITC-NAC at 23.75 min showed a distinct [M+Na]+ peak at 321 and 285 respectively, due to the addition of sodium bicarbonate to the sample, added to enhance the molecular ion.
Urine collection and analysis
Mice were housed in individual beakers and 24 h urine samples following oral administration of sinigrin were collected and the urine samples were stored at -20°C until analysis. The sample was analyzed for the mercapturic acid of allyl isothiocyanate (AITCNAC) by HPLC-MS system [20]. Briefly, 20 μL of an internal standard solution was added to 200 μL of urine in a 0.5 ml Eppendorf tube followed by vortexing. Then this mixture was filtered by HPLC filters (0.45 μm) and 10 μL was analyzed on an HPLC-MS system. Because the phenyl glucosinolate is not a naturally occurring compound, the mercapturic acid of phenyl isothiocyanate (PITC-NAC) was used as an internal standard (IS).
Measurement of the total GSH, GSH and GSSG level
Blood samples were centrifuged at 9,000 g for 3 min, and 10 μL serum was obtained for measurement total GSH, GSH and GSSG. The liver and pancreas were isolated after perfusion with ice-cold 1.15 % KCL and homogenized with 4 vol (w/v) 50 mM sodium phosphate buffer (pH 7.4) containing 1mM EDTA on ice. Then the homogenate was centrifuged at 10,000 g for 10 min at 4°C to take supernatant for measurement. The concentrations of total glutathione (in GSH equivalents) are measured using the kinetic assay [21]. The concentrations of GSSG are determined with 1M of 4-vinyl pyridine (4-VP) pretreatment for 1 h to mask GSH in the samples. The levels of GSH can be calculated from the difference in concentrations of the total and GSSG.
Enzyme activity of glutathione-S-transferase (GST) and gammaglutamyl transpeptidase (γ-GTP) in liver tissue
The supernatant of liver homogenate was used for the measurement of enzyme activities. The activity of GST was determined by using 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate. The reaction mixture contained 20 mM of CDNB and 20 mM reduced glutathione in 0.1 M sodium phosphate buffer (pH 6.5). The formation of the reduced GSH–CDNB conjugate was measured spectrophotometrically at 340 nm with CDNB [22]. γ-GTP was measured by using commercial reagent from the γ-GTP kit (Tokyo, Japan).
Evaluation of the free cysteine level in liver tissue
For analysis of free cysteine with pre-labeled HPLC method using gradient eluents A and B (Wako, Osaka), the liver homogenate was treated with a 5% MPA similar to precipitate protein. Cysteine standard and liver homogenate were derivatized at room temperature using a precolumn procedure. An LC-10AS HPLC system (Shimadzu, Tokyo, Japan) was equipped with an SCL-10A UV detector at 250 nm (λmax). Data were acquired and processed using Rec Pro software (Run time Instrument Co.). Samples were injected at 10 μL onto an ODS 18 column (250 × 4.6 mm, 5 μm i.d.) protected with a guard column. The column was operated at 40°C (CTO-10A, Tokyo, Japan) with a flow rate of 1 mL/ min using ACN/20 mM sodium phosphate buffer pH 6.0 (6:94) as eluent A and ACN/20 mM sodium phosphate buffer pH 6.0 (70:30) as eluent B. Identification was based on the comparison between the retention time of cysteine standard and those in the liver homogenate and was confirmed by a fortification technique (spiking). Quantitation was based on the external standard using calibration curves fitted by linear regression analysis. To assess the accuracy of the method, a recovery of the study was carried out by adding each cysteine at three concentration levels to a liver homogenate that was previously analyzed. The resulting fortified samples were analyzed in triplicate. The limit of the detection (LOD) was evaluated as three times the signal to noise ratio.
Statistically analysis
All measurements and analyses have been repeated a minimum of three times. Results were presented as means ± SE. Statistical significance was determined using a one-way analysis of variance (ANOVA) followed by the Tukey’s test, using SPSS version 22.0. Values of P<0.05 was considered statistically significant differences.
Effects of sinigrin on BW, DW, food consumption
The body weight gain in Figure 3A was significantly elevated in normal groups compared with diabetic groups (P<0.05). The body weight gain in the diabetic control group was significantly lower than these of the normal control group. After oral administration of sinigrin at a dose of 15 or 30 μmol/kg body weight/day for 21 days in diabetic groups, bodyweight gain increased significantly compared with those of the diabetic control group.
Figure 3: TG reduction (%) from T0 to T1 in risk groups for acute pancreatitis
As shown in Figure 3B and C, food and water intake in the diabetic control groups were increased dramatically compared with these of the normal groups. In the case of diabetic groups, the oral administration of 15 and 30 μmol sinigrin led to a decrease in food and water intake compared with these of the diabetic control group. Besides, the administration of sinigrin did not change the amount of food and water intake among normal groups.
Effect of sinigrin on glucose, triglyceride level, and insulin resistance
As shown in Figure 4, plasma glucose levels in diabetic control mice were significantly higher than those of normal control mice. The glucose level in diabetic control groups rose noticeably from 1 week after STZ administration and the high glucose levels were thereafter maintained until 21 days (the termination of this experiment) (initial: 147.40 mg/dL, 1 week: 453.37 mg/dL, 21 days: 639.54 mg/dL). These biological parameters were significantly decreased after the oral administration of sinigrin among diabetic mice. On the other hand, the declined level shown a dose-dependent decrease after the oral administration of sinigrin. In the diabetic mice were orally administered a low dose (15 μmol/kg BW) decreased the plasma glucose level from 505.30 to 264.20 mg/dL, that of a high dose (30 μmol/kg BW) was markedly decreased from 535.25 to 164 mg/dL in Figure 4. Other blood parameters for diabetic conditions were summarized in Table 1. After 21 days of the administration, the triglyceride level in diabetic groups treated with sinigrin was lower than the diabetic control group. Triglyceride levels in the diabetic group which treated at a low and high dose of sinigrin were 288.80 ± 19.18 and 171.13 ± 55.38 respectively compared to the high value 780 ± 75.63 in diabetic control groups.
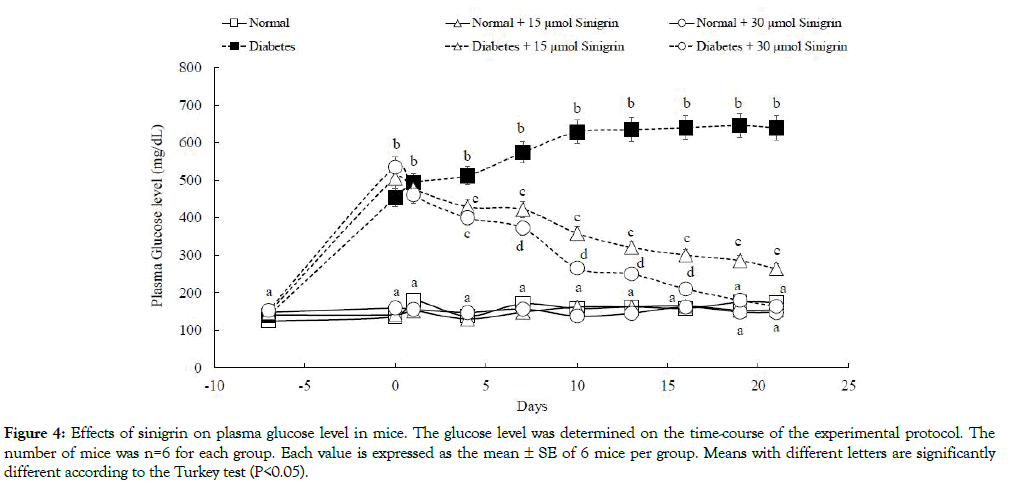
Figure 4: Effects of sinigrin on plasma glucose level in mice. The glucose level was determined on the time-course of the experimental protocol. The number of mice was n=6 for each group. Each value is expressed as the mean ± SE of 6 mice per group. Means with different letters are significantly different according to the Turkey test (P<0.05).
Table 1: Mean age, HbA1c and lipid profile in diabetic and non-diabetic patients at admission (T0).
| Group | Dose of sinigrin# | Triglyceride (mg/dL) | Glucose (mg/dL) | Insulin (ng/dL) | QUICKI | HOMA |
|---|---|---|---|---|---|---|
| Normal | 0 | 176.9 ± 40.6a | 174.3 ± 19.3a | 2.871 ± 0.036a | 0.87 ± 0.03a | 0.64 ± 0.03a |
| 15 | 175.4 ± 66.3a | 154.0 ± 16.9a | 2.954 ± 0.012a | 0.92 ± 0.04a | 0.53 ± 0.06a | |
| 30 | 171.1 ± 25.8a | 147.2 ± 19.3a | 2.961 ± 0.248a | 0.93 ± 0.08a | 0.55 ± 0.11a | |
| Diabetes | 0 | 780.0 ± 75.6b | 639.5 ± 9.3b | 2.650 ± 0.194a | 0.59 ± 0.01b | 2.17 ± 0.15b |
| 15 | 288.8 ± 19.2c | 264.2 ± 19.2c | 2.540 ± 0.327a | 0.78 ± 0.04ac | 0.86 ± 0.14ac | |
| 30 | 171.1 ± 55.4a | 164.0 ± 19.2a | 2.810 ± 0.421a | 0.90 ± 0.09a | 0.59 ± 0.14a |
Note: QUICKI is indices of insulin sensitivity whose value decreases with increasing insulin resistance. HOMA is an index of insulin resistance whose value increases with increasing insulin resistance. QUICKI = 1/[log(I0) + log(G0)]; HOMA = (I0 x G0)/22.5 (Glucose concentration in mM). Each value was expressed as the mean ± SE of 6 mice per group. Mean with different letters are significantly different according to the Turkey test (P<0.05). #: the dose of sinigrin (µmol/kg BW/day).
Non-fasting plasma insulin levels in the diabetic control mice decreased non-significant differences compared to the normal control mice which induced the increasing the glucose levels, in part, due to the progression of insulin resistance in T2D [23]. Besides, HOMA and QUICKI are indexes of insulin resistance whose value was significantly different in diabetic control groups compared to normal groups. On the other hand, the administration of the sinigrin compound has no significant difference in the HOMA and QUICKI at the normal groups. However, the treatment with a low dose and high dose sinigrin resulted in a significant reduction of HOMA and increasing of QUICKI, indicating a marked improvement in insulin sensitivity by both doses in the T2D mice.
Allyl isothiocyanate metabolism level in plasma and tissue
After 21-day treatment of sinigrin, mice have collected the whole blood, liver, and pancreas for the determination of AITC level absorption and distribution. Our data indicate that the amount of AITC detected in plasma was significantly different between normal control groups and diabetic control groups although they intake the same dose of sinigrin. In deep, the AITC metabolite level in the group of normal mice was 0.21 ± 0.02 and 0.49 ± 0.06 nmol/mL at the dose of 15 and 30 μmol respectively. In contrast, the diabetes mice resulted in only 0.08 ± 0.03 and 0.15 ± 0.02 nmol/mL following administration of a low and high dose sinigrin in Figure 5A. Both liver and pancreas, we observed the level accumulation was significantly higher 7- 10 times in the T2D mice compared to normal mice in Figure 5A. Specifically, this is the first report to evaluate AITC accumulation in the pancreas. Our data illustrated that in the T2D mice, AITC inclined to accumulate into the pancreas. Importantly, these results are corroborated by the improvement of sinigrin treatment on the T2D mice model. None AITC was found in the group without treatment of sinigrin.
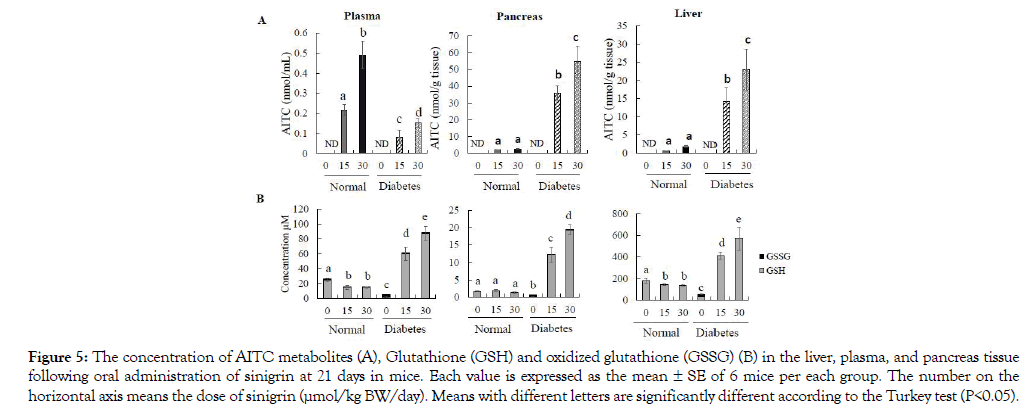
Figure 5: The concentration of AITC metabolites (A), Glutathione (GSH) and oxidized glutathione (GSSG) (B) in the liver, plasma, and pancreas tissue following oral administration of sinigrin at 21 days in mice. Each value is expressed as the mean ± SE of 6 mice per each group. The number on the horizontal axis means the dose of sinigrin (μmol/kg BW/day). Means with different letters are significantly different according to the Turkey test (P<0.05).
Effects of sinigrin on glutathione level
In the diabetic control group, we observed the low level of GSH and total GSH reversely with the highest concentration of GSSG in all of the measurements of plasma, liver, and pancreas tissue compared to those of normal control mice (P<0.05) in Figure 5B. In the plasma and liver tissue, we observed the dose-dependent increase of GSH and total GSH levels in diabetes with oral administration of sinigrin at both low and high doses (15 and 30 μmol/21 days). Among the group of normal mice, the treatment of sinigrin has a decline in the level of GSH and total GSH compared to normal control mice. Besides, GSSG was not significantly different between normal and diabetes groups treated with the sinigrin compound in Figure 5B. In the pancreas tissue, oral administration of sinigrin also shown the highest concentration of GSH and total GSH levels in the diabetic mice compared to the normal control mice in Figure 5B. Remarkably, the level of GSH in the normal mice treated with sinigrin did not decline as in the case of plasma and liver tissue. The trend of GSSG levels is like plasma and liver tissue. Thus, increasing GSH levels were observed in plasma, liver, and pancreas tissue among the T2D mice which orally administered of sinigrin at low and high dose (15 and 30 μmol/21 days). This data suggested that sinigrin enhances the GSH levels in the T2D condition compare to the severe GSH level in the T2D control group.
Urinary of AITC-NAC conjugate after sinigrin treatment
After oral administration of sinigrin (15 μmol or 30 μmol), urine was collected in 24h. After analysis of chromatography, the peak of AITC-NAC was found in the urine samples of the sinigrin treated group with the corresponding mass (m/z 263 and 285) and retention time at 23.75 min identified as the peak of synthesis AITC-NAC standard (Figure 6). There was no detectable amount of ITC conjugate in the group without sinigrin treatment (Figure 6). A standard curve was constructed in the linearity range (0.09 – 23.85 nM, R2= 0.996). The percentage of recovery of AITC-NAC was calculated base on the standard of the synthetic of AITCNAC and quantitated using PITC-NAC as the internal standard. Recovery of AITC-NAC was excreted in urine revealed significantly different in normal and type 2 diabetic mice Table 2. In normal mice, the recovery of AITC-NAC accounted for 70% of the oral dose. There was no dose-dependence between 15 and 30 μmol sinigrin. However, in T2D the percentage of mercapturic acid AITC-NAC was found only approximately 10%. This metabolized marker AITC-NAC suggested low exposure in T2D compared to healthy mice.
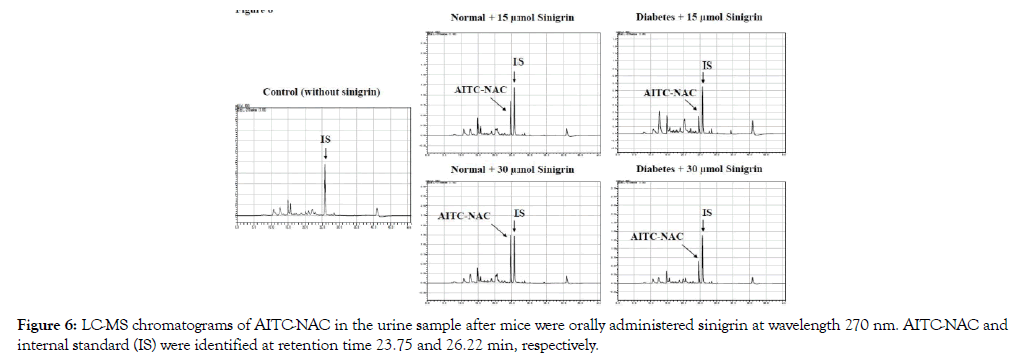
Figure 6: LC-MS chromatograms of AITC-NAC in the urine sample after mice were orally administered sinigrin at wavelength 270 nm. AITC-NAC and internal standard (IS) were identified at retention time 23.75 and 26.22 min, respectively.
Correlation of excretion of AITC-NAC and other factors in the metabolism pathway of sinigrin
These parameters were summarized in Table 2. There was higher significantly GST activity in the liver tissue of sinigrin-treated normal mice than in those of normal control mice (P<0.05). In contrast, the GST activity was not induced in diabetic mice who orally administered sinigrin at both doses (15 and 30 μmol). STZmediated increased the GST activity in the mouse liver observed in the diabetic control mice.
Table 2: Correlation of excretion of AITC-NAC and other factors in the metabolism pathway of sinigrin.
| Group | Dose of Sinigrin# | AITC-NAC % | Liver tissue | ||||
|---|---|---|---|---|---|---|---|
| AITC (nmol/g tissue) | GSH (µM) | Cysteine (nmol/g tissue) | GST (nmol/min/mg protein) | g-GTP (nmol/min/mg protein) | |||
| Normal | 0 | Nd | Nd | 238.49 ± 20.83a | 86.42 ± 10.81a | 764.27 ± 152.06a | 19.21 ± 3.94a |
| 15 | 68.69 ± 10.50a | 0.53 ± 0.11a | 143.40 ± 6.72b | 57.27 ± 6.39b | 1185.87 ± 222.07b | 55.21 ± 9.72b | |
| 30 | 70.22 ± 7.45a | 1.77 ± 0.26a | 135.79 ± 7.29b | 50.73 ± 6.44b | 1239.89 ± 177.15b | 77.26 ± 7.85c | |
| Diabetes | 0 | Nd | Nd | 31.29 ± 8.00c | 20.60 ± 12.17c | 1109.37 ± 123.82b | 101.86 ± 6.45d |
| 15 | 5.12 ± 4.12b | 14.16 ± 3.79b | 410.34 ± 35.11d | 115.85 ± 12.79d | 764.46 ± 173.24a | 26.05 ± 3.64a | |
| 30 | 6.82 ± 5.11b | 22.98 ± 5.70c | 570.35 ± 106.24e | 151.45 ± 17.05e | 728.64 ± 179.45a | 20.09 ± 2.34a | |
Note: AITC-NAC was measured after 24h oral administration of sinigrin in all groups of this experiment. Glutathione (GSH), AITC levels, cysteine levels, GST, and -GTP enzyme activity were determined in all groups of this experiment at the day 21st. Each value is expressed as the mean ± SE of 6 mice per group. Means with different letters are significantly different according to the Turkey test (P<0.05). #: the dose of sinigrin (µmol/kg BW/day).
The results showed that γ-GTP increased at the highest level in the control diabetes group compared to the normal control group (P<0.05). Treatment of sinigrin in normal groups elevated significantly the levels of γ-GTP compare to the normal control group. On the other hand, in the T2D mice, supplementation of sinigrin was like those of normal control mice.
The cysteine levels were measured in the liver tissue of all mice groups and results were shown in Table 2. The lowest cysteine content was in the type 2 diabetic control group compared to those of normal control mice (P<0.05). Supplement of sinigrin in the normal mice leads to a decline in cysteine levels compared to those of normal control mice. However, these values were not significantly suppressed as in the case of type 2 diabetic control groups. In diabetes plus sinigrin 15 and 30 μmol/kg BW group, the cysteine values were significantly elevated approximately 1.5 and 2 times higher than those of diabetic control mice (P<0.05).
Our study is focused on the anti-diabetic effects of GLS and the metabolite ITC following the daily intake of purified sinigrin.
The current study conducted using purified GLS on the experimental diabetic model clearly shows that daily intake of sinigrin exhibits anti-diabetic activities in type 2 diabetic mice. The changes in BW gain with food and water consumption due to metabolic failure in diabetic conditions [24] were perfectly recovered after sinigrin treatment (Figure 3). And sinigrin improved insulin resistance (IR) by decreasing the plasma glucose and triglyceride levels (Figure 4). IR is a major determinant of the markedly increased risk of cardiovascular complications in type 2 diabetic patients [25]. Treatment of IR is a potential target for the improvement of hyperglycemia and a decrease in cardiovascular disease risk of factors [26]. In the current study, a 21-day supplementation with 15 and 30 μmol sinigrin improved the IR index including both HOMA and QUICKI indexes (Table 1). Therefore, our data proved that the consumption of sinigrin (GLS form) possess antidiabetes activity. The intake amount of sinigrin in the current mice experiment was 6-12 mg/day is approximately equal to 200- 400 g of Brassicaceae vegetables per day (In broccoli, sinigrin is approximately 3 mg/100 g vegetables). In another clinical study, the oral administration of broccoli extract at dose 200 mg/kg BW/ day for 20 days improved the plasma glucose level [10]. Another study in diabetic patients has resulted indicated the sulforaphane (ITC form) in broccoli sprouts powder (10 g/day) has an impact on the improvement of the IR index [11]. Besides, the GOT and GPT levels were not significantly different between the sinigrin treated normal mice and normal control mice in our experimental condition (data not shown). Although γ-GTP activity slightly increased, these results are thought that sinigrin did not cause a bad effect on the healthy mice in our condition. Based on these data, effective consumption amount of sinigrin can cause a good effect on suppression T2D. In our research, we demonstrated the protective effect of sinigrin on the improvement of the T2D mice by reducing plasma glucose levels and improving insulin resistance.
These therapeutic activities in vivo are contributed by absorbed AITC generated by myrosinase from dietary sinigrin. After tissue distribution, AITC is conjugated to glutathione in the liver and excreted in the urine as mercapturic acid (AITC-NAC), which accounts for a 50-80% dose of ingestion ITC [27-29]. In the current study, the AITC-NAC equivalent to approximately 70% of intakes sinigrin was excreted in the urine in the normal mice Table 2. This value revealed that the amount of AITC in the body whether mice were orally administered pure GLS (sinigrin). The AITC is known as an activator of transcription factor NF-E2-related factor 2 (Nrf2) through its capacity to induce the expression of phase 2 enzymes that detoxify reactive oxygen species and other antioxidant proteins [30,31]. Data in the normal mice revealed that the intake of sinigrin enhanced the GST activity in liver tissue compared to the normal control mice. This data has an agreement with the previous report about the supplementation of GLS compounds lead to induce the phase 2 enzyme activity as GST [30]. In response to GST enhancement by sinigrin, the conjugate metabolism of sinigrin declined the level of GSH and excrete a lot of AITC-NAC (Table 2). Finally, sinigrin was lead to falling the total GSH level in normal mice (Figure 5B). This result has an agreement that AITC consumed GSH for producing the AITC-NAC. Thus, the tissue GSH level has a negative-corresponding with the excretion level of AITC-NAC in the urine.
To estimate the mechanism of anti-diabetic effects of sinigrin in T2D mice, we have analyzed several parameters related to the metabolic pathway of AITC in mice body. We found big differences in parameters between normal and diabetic condition and it will contribute improve the diabetic condition. Additionally, in contrast to normal mice excreting of 70% AITC-NAC in urine, we found that the elimination of AITC-NAC was very low in the urine of diabetic mice, approximately 6% corresponded to intake sinigrin (Table 2). This evidence suggests that the amount of sinigrin excretion is less than the percentage expose in the healthy mice. In diabetic control mice, we resulted in the significant enhancement of GST activity compared to normal control mice. However, in the diabetic mice unexpectedly, the supplementation of sinigrin did not enhance the GST enzyme activity in the liver tissue. In the previous studies with a long period observation after STZ injection for an increase in GST activity to appear suggested that the development of the diabetic state might be responsible for this increase, with the claim of the positive relationship between increasing the blood glucose level and elevating GST activity in the diabetic state. As the treatment of sinigrin improves the suppression of blood glucose level as mentioned above (Figure 4), we can estimate that sinigrin's impact on the metabolism pathway of AITC including GST may contribute to their activity in diabetic disease.
The bioavailability of ITC might contribute to their biological activity. The pharmacokinetic studies of ITC metabolism and distribution in mice following a single dose and long-term observation of the distribution of ITC have published, which reported the metabolite profile in plasma and various tissue [27-29]. Until now, no research reported the amount of metabolite after oral administration of GLS in the model disease. In the current study, we analyzed the AITC metabolism level in plasma and tissue in T2D mice by oral administration of sinigrin. Our data indicated that AITC accumulates at a high level in the pancreas and liver tissue of T2D mice compare to healthy mice following the same treatment of sinigrin. After dietary sinigrin, AITC metabolites tend to store in these tissues. As we know, both are critical target tissues to investigate the distribution of sinigrin in T2D.
Interestingly, the amount of elimination AITC-NAC in urine and accumulation of AITC in the liver and pancreas tissue showed a negative correlation. In deep, the distribution of AITC is located more in the pancreas compared to the liver which reported as the primary tissue for the metabolism of AITC. The data evidenced that AITC is inclined to distribute more in the target tissue as a pancreas under T2D condition. In deep, the amount of AITC distributes in the liver and pancreas is 1.7 and 2.7 nmol/g tissue respectively in the normal mice. However, the observed high value was detected at 22 and 54 nmol/g tissue in the liver and pancreas of T2D mice in Figure 5A. Increased accumulation of AITC in these tissues illustrates the changeable metabolism of sinigrin in T2D. In our research, we demonstrated the protective effect of sinigrin on the improvement of the T2D mice by reducing plasma glucose levels and improving insulin resistance. Therefore, the high accumulation of AITC may collaborate with the result associating intake sinigrin protect Type 2 diabetes.
The high level of AITC-NAC excretes corresponds to consume a high amount of GSH. According to Xu et al., 2001, incubation of Phenyl Isothiocyanate (PEITC) significantly decrease the total GSH and GSH level in human leukemia cells in vitro due to conjugated reaction of PEITC with GSH catalyzed by GST, these reactions may lead to a temporally release in cysteinyl thiol groups in cells that provide an oxidative trigger for apoptosis [32]. In our study, the treatment of sinigrin also declined the level of GSH, leading to falling the total GSH level in normal mice in Figure 5B. This result has an agreement that AITC consumed GSH for producing the AITC-NAC. Interestingly, a fall of GSH level did not reach the peak value of diabetic control mice. Also, the oxidized GSSG level was like healthy mice. Therefore, although the treatment of sinigrin declined the GSH level, the intake of sinigrin did not harm the growth of mice following the time-course of the experiment as the evidence of enhancing the body weight gain (Figure 3A).
On the other hand, GSH may expose the oxidation via enhanced free radical generation in diabetic conditions [33]. In type 2 diabetes, dysfunctional redox homeostasis has long been known to play a role in the pathogenesis of the disease and its complications through a variety of mechanisms, and diabetic patients have been shown to possess increased cellular levels of reactive oxygen species (ROS) and ROS-induced DNA damage [34]. GSH pathway impairs in the diabetic condition and increases the susceptibility of the GSH pathway to oxidation in type 2 diabetic patients [35]. Notably, in the diabetic mice treated sinigrin, the total GSH and GSH levels were significantly higher than those of normal mice (Figure 5B). This result correlates to the low expose AITC-NAC in diabetic mice (Table 2). Remarkably, In the diabetic mice treated sinigrin, the total and GSH levels were dramatically higher than the normal mice without treatment of sinigrin. Our data is the first reports showed the supplement of sinigrin impact on the enhancing GSH level. It also clearly indicates the protective effect of the dose-dependence of sinigrin on GSH production.
GSH is a tripeptide made up of the amino acids glutamine, cysteine, and glycine. These amino acids are vital for the maintenance of circulating and tissue levels of GSH. We see that the cysteine was used to produce the AITC-NAC as the biomarker of intake of sinigrin. Therefore, the metabolism of sinigrin may decrease the level of cysteine. In our data, the results indicated that sinigrin declines cysteine levels in normal mice (Table 2). The lowest cysteine level was evaluated in the diabetic control groups. These results have an agreement in another previous study, which cysteine level is often found to be lower in diabetes [36,37]. Remarkably, the supplementation of sinigrin significantly enhances the levels of cysteine around 1.5 and 2 times at the dose of 15 and 30 μmol sinigrin/kg BW. This data might be correlated to the low expose of AITC-NAC and restore the cysteine levels leading to elevating the cysteine concentration in the diabetic mice model (Table 2). Interestingly, we observed that the dietary of sinigrin increase the level of glutathione (GSH) in both plasma, liver, and pancreas tissue in Figure 5B. In the previous study in patients, this suggests that cysteine status directly and positively influence the GSH levels. Sufficient nutritional amounts of vitamin D and cysteine independently can increase levels of GSH and thereby decrease insulin resistance levels in T2D patients. Finally, sinigrin compound proved a positive effect on T2D, in terms of saving natural antioxidant GSH lead to protect the oxidative condition in T2D mice.
It was summarized in Figure 7 about the metabolism pathway of sinigrin. As shown in the current research, the ITC metabolizing system was changed to protect oxidative stress in the diabetic condition in vivo. Here we see that the cysteine was used to produce the AITC-NAC as the biomarker of intake of sinigrin. Based on comparison to normal conditions, we analyzed the relationship between exposure AITC-NAC in urine and other metabolized related factors. Oral administration of sinigrin in diabetic mice showed a significant increase of GSH, cysteine level, and high accumulation of AITC levels in liver tissue. In contrast, we observed that among the diabetic mice treated with sinigrin led to a decline in the GST and γ-GTP activity. Besides, our data observed the low excretion of AITC-NAC in T2D mice. Thus, these results suggested that sinigrin treatment in T2D mice less excreted AITCNAC because it caused the impact on the metabolism pathway to save GSH to improve the diabetic condition.
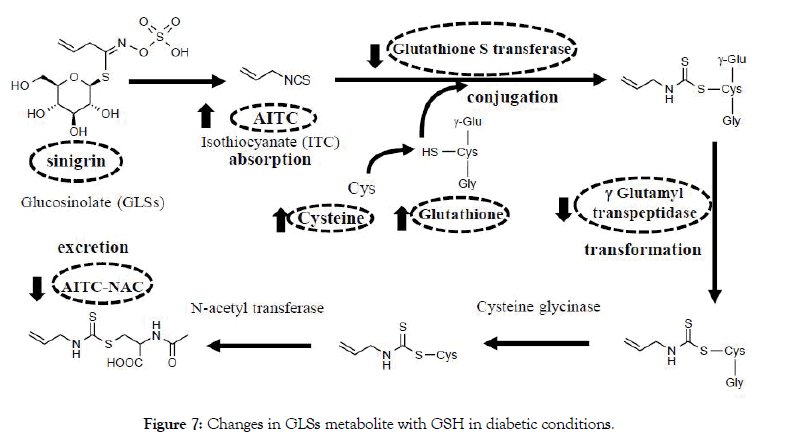
Figure 7: Changes in GLSs metabolite with GSH in diabetic conditions.
In conclusion, our study indicated that daily intake of sinigrin decreased plasma glucose, triglyceride levels, and improved insulin resistance in type 2 diabetic mice. Besides, the treatment of sinigrin reveals the decline of urinary marker AITC-NAC in type 2 diabetic conditions compared to healthy mice. Sinigrin treatment inclined to accumulate at a high-level of AITC in the liver and pancreas accompanied by accumulation of GSH. Daily intake of sinigrin enhancing the GSH levels in pancreas tissue leading to improve oxidative status in the T2D mice. Thus, metabolize change was improved insulin resistance and suppress oxidative stress in diabetic conditions. Therefore, these findings propose that sinigrin can serve as a novel therapeutic agent for T2D.
This study was supported by the Department of Food Chemistry and Functional Nutrition, Graduate School of Marine Science and Technology, TUMSAT University. This study is funded in part by the Can Tho University Improvement Project VN14-P6, supported by a Japanese ODA loan.
The authors declare that there is no conflict of interests regarding the publication of this paper.
Citation: Truong TTP, Koyama T (2020) Glucosinolate Sinigrin Improves Insulin Resistance to Suppress Glutathione Consumption in Type 2 Diabetic Mice. J Diabetes Metab. 11:859. doi: 10.35248/2155-6156.20.11.859.
Received: 08-Aug-2020 Published: 25-Oct-2020
Copyright: © 2020 Truong TTP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.