Research Article - (2020) Volume 11, Issue 8
Background: Young-onset type 2 diabetes (T2DMY) is considered to have a more aggressive disease phenotype, unfavourable long-term outcomes, and a close association with low-grade chronic inflammation. The aim of this study was to explore the circulating inflammatory profile in a cohort of adults with T2DMY and assess its potential associations.
Methods: T2DMY adults (n=65) and non-diabetic controls (n=40) underwent detailed anthropometric and biochemical characterisation. Serum levels of six pro-inflammatory biomarkers (soluble E-selectin, IL-1β, IFN-γ, IL-6, IL-8 and angiopoietin like protein-4) were measured by Magnetic Luminex Assays and were compared between study groups. Potential associations of these biomarkers with anthropometric/biochemical parameters and microvascular complications were also tested in the T2DMY group.
Results: T2DMY patients had significantly higher circulating levels of all measured inflammatory markers compared to controls, even after adjusting for age and gender (E-selectin: 65.9 ± 4.5 vs. 37.9 ± 8.2 ng/ml; IFN-γ: 220.9 ± 7.4 vs. 160.6 ± 13.5 pg/ml; IL1-β: 50.1 ± 1.8 vs. 38.0 ± 3.3 pg/ml; IL-6: 17.5 ± 0.7 vs. 11.4 ± 1.1 pg/ml; IL-8: 18.1 ± 1.5 vs. 11.7 ± 2.4 pg/ ml; ANGPTL-4: 162.8 ± 9.8 vs. 109.0 ± 15.6 ng/ml; all p-values<0.05). Significant positive correlations were found between fat mass and IL-6 and ANGPTL-4; waist-to-hip ratio and E-selectin, IFN-γ, IL-1β and IL-6; and HbA1c and E-selectin, IFN-γ, IL-1β and IL-6. Within the T2DMY group, E-selectin was significantly elevated in patients with combined albuminuria and retinopathy compared with those without these complications (88.97 ± 43.59 vs 59.38 ± 25.28 ng/ml; p=0.003).
Conclusions: T2DMY patients exhibit an adverse profile of key circulating pro-inflammatory biomarkers, which appears associated with worse anthropometric/biochemical parameters and microvascular complications. The role of these biomarkers as predictors of T2DMY and disease progression should be further elucidated.
Young-onset type 2 diabetes; T2DMY; Soluble E-selectin; IL-1β; IFN-γ; IL-6, IL-8; Angiopoietin like protein-4; ANGPTL-4
The number of people living with type 2 diabetes (T2DM) has now reached approximately 463 million around the world and the prevalence of the disease in the 20-39 years age group is estimated to be 12% in women and 13% in men [1]. The growing number of young adults with T2DM poses a clinical challenge as these patients have potentially longer disease exposure, an increased propensity to more rapidly develop adverse cardiovascular diseases (CVD) and reduced life expectancy [2]. Although the mechanisms leading to T2DMY development are similar to those for T2DM in older patients, there might be distinct interactions and mechanisms implicated in the metabolic dysfunctions of T2DMY [3].
The link between metabolic dysregulation and inflammation is well established, [4] and high levels of circulating inflammatory markers have been closely associated with the development and progression of T2DM and CVD [5]. In adults, the documented correlation between inflammation and T2DM indicates that underlying chronic low-grade inflammation may contribute to insulin resistance, metabolic disorders and T2DM complications (e.g. CVD or kidney disease) [5,6]. Moreover, the identification of potential pro-inflammatory pathways involved in the development and progression of T2DM has led to a growing interest in studies targeting inflammation to improve insulin secretion and sensitivity [7]. Chronic hyperglycaemia is known to affect β-cell mass and function via pancreatic islet inflammation and/or via islet endothelial cell alterations [3]. Amongst several pro-inflammatory cytokines identified within islets, interleukin 1β (IL-1β) appears to play a fundamental role in the initiation and amplification of β-cell inflammation and dysfunction [8]. Moreover, IL-1β activity is enhanced by other cytokines originating from inflammatory processes in the adipose tissue, which could further disrupt the equilibrium of circulating cytokines [8]. For instance, interferon-γ (IFN-γ), a cytokine expressed by natural killer cells and T-lymphocytes, plays a significant role in activating macrophages, which, in turn, are implicated in complications such as atherosclerosis [9]. Other key cytokines expressed from macrophages include interleukin-6 (IL-6), interleukin-8 (IL-8) and angiopoietin-like protein-4 (ANGPTL-4). Indeed, IL-6 is thought to regulate glucose metabolism by interfering with insulin signalling in hepatocytes [10], whereas in patients with obesity IL-6 may contribute to T2DM pathogenesis by both interfering with insulin signalling and impairing β-cell function [3]. Similarly, IL-8 is a chemokine associated with impaired insulin sensitivity, whilst it also promotes poor metabolic and lipid profiles through effects on vascular smooth muscle cells, endothelial cells and macrophages [11]. Furthermore, ANGPTL-4 is now recognized as a key regulator of lipoprotein metabolism with high expression in the liver and adipose tissue, and has been directly associated with the risk of T2DM and atherosclerosis [12-14]. Finally, the links between metabolic dysregulation, chronic inflammation and increased CVD morbidity in T2DM patients further involve vascular endothelium dysfunction [15]. As such, E-selectin, which is a crucial endothelial adhesion molecule that is expressed and released from cytokineactivated endothelial cells, represents an endothelium injury/ atherosclerosis biomarker with an important role in predicting CVD [16].
Although increased circulating levels of the aforementioned pro-inflammatory biomarkers have been reported in older adult patients with T2DM [3,5], there is still a paucity of relevant evidence in adults with youth-onset type 2 diabetes mellitus (T2DMY). A limited number of clinical studies exploring the association between inflammation and T2DM in children and adolescents have demonstrated that chronic low-grade inflammation pathways can adversely affect metabolic homeostasis and T2DM development [17-20]. Considering that effects noted during adolescence can, at least in part, persist in early adulthood, inflammation is considered to play an important role in T2DM predisposition during early adulthood. The latter may further contribute to the rising T2DM prevalence in younger age groups [1] and their increased predisposition to diabetes-related complications [21].
The aim of the present study was to characterize the circulating profile of the aforementioned pro-inflammatory biomarkers in a cohort of adult T2DMY patients and explore potential associations with diabetes-related microvascular complications.
Study Population
We recruited a cohort of 100 adults diagnosed with young-onset type 2 diabetes mellitus. T2DMY patients included in this study were aged at least 18 years old, had a T2DM diagnosis before the age of 40 years and were able to provide informed consent (Figure 1). Key exclusion criteria were known diagnosis of type 1 diabetes or secondary diabetes, diabetes duration over 15 years, and any other significant non-diabetes related illness/condition (e.g. cancer, autoimmune, psychiatric or developmental disorders). T2DMY patients enrolled in the study were asked to provide consent for blood sampling for additional investigations (e.g. assessment of pro-inflammatory profiles). As such, for the purposes of this study, circulating inflammatory markers were measured in consented T2DMY patients (N=65) without established CVD (e.g. history of myocardial infarction, stroke and revascularisation procedures), since diagnosed CVD could have further affected the serum levels of the assessed inflammatory markers [22]. To enable comparison with healthy non-diabetic controls, we also recruited a group of 40 healthy volunteers aged between 20-40 years. Diabetes was excluded in each of these healthy volunteers based on both HbA1c (<39 mmol/mol) and fasting plasma glucose (<5.6 mmol/L) measurements. The study was approved by the West Midlands National Research Ethics Service (REC reference number:12/WM/0166) and all participants provided written informed consent. A flowchart of the study recruitment process, including the key inclusion and exclusion criteria, is presented in Figure 1.
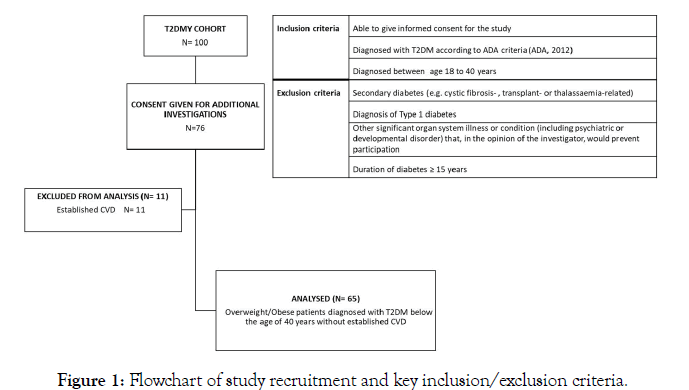
Figure 1: Flowchart of study recruitment and key inclusion/exclusion criteria.
Anthropometric assessments and routine biochemistry All study participants underwent detailed assessments including: demographic details; medical and medication history. Body mass index (BMI; kg/m2) was calculated as the ratio of weight in kilograms (kg) by the height squared in meters (m2). Body composition was also measured using an automated bio-impedance analyser (Tanita BC 420 S MA). Routine biochemical assessments after overnight fasting included tests for lipid profile, renal function, liver function, thyroid function, vitamin D and B12, fasting plasma glucose, and haemoglobin A1c (HbA1c). Moreover, microalbuminuria status was classified as albumin/creatinine ratio >3.5 mg/mmol in females and >2.5 in males. Finally, peripheral neuropathy was assessed using the Michigan Neuropathy Screening Instrument (MNSI).
Measurement of selected circulating pro-inflammatory biomarkers
Serum levels of E-selectin, IFN-γ, IL-1 β, IL-6, ANGPTL-4 and IL-8 were analysed in duplicate using a Magnetic Luminex Assay (R&D systems, USA) as per the manufacturers’ protocol. These markers were selected based on the existing relevant data in older populations with T2DM and offer a basis for comparison with this T2DMY cohort. Serum detectable ranges for these measured biomarkers were as follows: E-Selectin: 1276-81670 pg/ml; IFN-γ: 222-14210 pg/ml; IL-1β: 74-4740 pg/ml; IL-6: 17-1100 pg/ml; ANGPTL-4: 7-438 pg/ml; and IL-8: 16-1060 pg/ml.
Statistical analysis was performed to identify differences in the inflammatory profile of the T2DMY patients in relation to gender, body fat distribution, glycaemic control and microvascular complications (retinopathy, albuminuria and peripheral neuropathy). Differences in the inflammatory markers between the two study groups were analysed using analysis of covariance (ANCOVA) with age incorporated as the continuous covariate. A Pearson coefficient was used to study the correlation between the inflammatory markers and anthropometric measurements and glycaemic control. The strength of the correlations was interpreted as follows, for absolute values of r: 0.2-0.39 as weak, 0.40-0.59 as moderate and 0.6-0.79 as strong [23]. Students’ t-test was used to compare circulating levels of inflammatory markers among T2DMY patients in the presence or absence of microvascular complications. All data were analysed using SPSS for Windows version 24.0 (SPSS Inc. Chicago, USA).
The clinical and biochemical characteristics of all study participants (65 T2DM patients and 40 volunteer non-diabetic controls) are presented in Table 1.
Table 1: Selected clinical and biochemical characteristics of the two study groups, namely patients with young-onset type 2 diabetes mellitus (T2DMY; N=65) and healthy non-diabetic controls (N=40).
| Variables | T2DMY | Controls | ||
|---|---|---|---|---|
| (N=65) | (N=40) | |||
| Male gender N (%) | 25 (38.5) | 11 (27.5) | ||
| Age (years) | 39.4 ± 6.9 | 31.4 ± 5.6 | ||
| Age at diagnosis with T2DM | 31.9 ± 5.4 | N/A | ||
| BMI (kg/m2) | 35.8 ± 9.4* | 24.0 ±3.7 | ||
| Fasting plasma glucose (mmol/L) | 9.8 ± 3.8* | 4.7 ± 0.4 | ||
| HbA1c (mmol/mol) | 73.3 ± 22.3* | 33.8 ± 5.1 | ||
| Systolic blood pressure (mmHg) | 123.9 ± 15 | 119.6 ± 15.5 | ||
| Diastolic blood pressure (mmHg) | 73.7 ± 9.5 | 77.2 ± 9.4 | ||
| Total Cholesterol (mmol/L) | 4.4 ± 1.1 | 4.8 ± 0.9 | ||
| LDL-cholesterol (mmol/L) | 2.4 ± 1.1 | 2.9 ± 0.8 | ||
| HDL-cholesterol (mmol/L) | 1.1 ± 0.4 | 1.5 ± 0.3 | ||
| Cholesterol/HDL ratio | 4.4 ± 1.3 | 3.2 ± 0.8 | ||
| Triglycerides (mmol/L) | 2.0 ± 1.0* | 0.9 ± 0.3 | ||
| GFR (ml/min) | 89.4 ± 2.3 | 85.5 ± 7.5 | ||
| Insulin treatment N (%) | 40 (61.5) | N/A | ||
| Lowering-lipid treatment N (%) | 42 (64.6) | N/A | ||
| Anti-hypertensive treatment N (%) | 33 (50.8) | N/A | ||
| Microvascular complications N (%): | ||||
| Retinopathy | 22 (33.8) | N/A | ||
| Albuminuria | 20 (30.8) | |||
| Peripheral neuropathy | 22 (33.8) | |||
Data are presented as mean ± standard deviation, unless otherwise specified; Lipid-lowering treatment includes statins, fibrates and ezetimibe; *significant compared to control at the level of p=0.05. BMI: body mass index; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; GFR: glomerular filtration rate; LDL: low-density lipoprotein
As expected, compared to controls and adjusted for age, T2DMY patients had higher fasting plasma glucose, HbA1c and BMI (Table 1). Specifically, none of the T2DMY patients had a BMI within the normal range, with 20% (N=13) being overweight ((BMI: 25 to 30 kg/m2) and 80% (N=52) with obesity (BMI>30 kg/m2). Overall, glycemic control in T2DMY patients was poor (mean HbA1c: 73.3 ± 22.3 mmol/mol) and 73.8% of them had a HbA1c above 53 mmol/mol. The majority of T2DMY patients (64.6%, N=42) had already developed at least one microvascular complication, namely retinopathy (33.8%, N=22), albuminuria (30.8%, N=20) and neuropathy (33.8%, N=22).
T2DMY patients had significantly higher circulating levels of all the six measured inflammatory markers compared to controls, and this remained significant even after adjusting for age and gender (Table 2). In patients with T2DMY, no significant associations were noted for these inflammation markers in relation to gender or age at diagnosis. However, there was a significant association between body fat mass and distribution and the assessed pro-inflammatory profile of T2DMY patients. Specifically, low to moderate positive correlations were observed between fat mass and IL-6 (r=0.28, p=0.03), and ANGPTL-4 (r=0.32, p=0.02), whilst E-selectin (r=0.41, p<0.001), IFN-γ (r=0.43, p<0.001), IL-1β (r=0.47, p=<0.001) and IL-6 (r=0.39, p=0.002) exhibited a moderate positive correlation with the waist-to-hip ratio. A significant positive association was also noted between HbA1c and E-selectin (r=0.46, p<0.001), IFN-γ (r=0.44, p<0.001), IL-1β (r=0.38, p<0.001) and IL-6 (r=0.30, p=0.002).
Table 2: Circulating levels of key pro-inflammatory biomarkers in the two study groups, namely patients with young-onset type 2 diabetes mellitus (T2DMY; N=65) and healthy non-diabetic controls (N=40).
| BIOMARKER | T2DMY | CONTROLS | p* |
|---|---|---|---|
| E-selectin (ng/ml) | 65.9 ± 4.5 | 37.9 ± 8.2 | 0.008 |
| IFN-γ (pg/ml) | 220.9 ± 7.4 | 160.6 ± 13.5 | 0.001 |
| IL1-β (pg/ml) | 50.1 ± 1.8 | 38.0 ± 3.3 | 0.004 |
| IL-6 (pg/ml) | 17.5 ± 0.7 | 11.4 ± 1.1 | <0.001 |
| IL-8 (pg/ml) | 18.1 ± 1.5 | 11.7 ± 2.4 | 0.041 |
| ANGPTL-4 (ng/ml) | 162.8 ± 9.8 | 109.0 ± 15.6 | 0.009 |
Comparisons adjusted for age and gender; *significant compared to control at level of p = 0.05. IFN-γ: IFN-gamma; IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; IL-8: Interleukin-8; ANGPTL-4: Angiopoietin-like protein 4
Within the T2DMY group, inflammatory markers were found to be higher in T2DMY patients with retinopathy and albuminuria compared to those without these complications, although this difference was not statistically significant (Table 3). However significant elevated levels of E-selectin were seen in T2DMY patients with combined albuminuria and retinopathy compared with those without any of these two complications (88.97 ± 43.59 vs 59.38 ± 25.28 ng/ml; t=3.09, p=0.003). Finally, patients with peripheral neuropathy showed significantly lower levels of serum ANGPTL-4 compared to those without (116.83 ± 57.95 vs 172.97 ± 83.99 ng/ml, respectively; t=2.74, p=0.008).
Table 3: Comparison of circulating levels of inflammatory markers in patients with and without microvascular disease (retinopathy, albuminuria and peripheral neuropathy).
| E-Selectin ng/ml | IFN-γ pg/ml | IL-1β pg/ml | IL-6 pg/ml | ANGPTL-4 ng/ml | IL-8 pg/ml | |||
|---|---|---|---|---|---|---|---|---|
| Retinopathy | ||||||||
| Yes (N=22) | 74.5 ± 37.2 | 221.5 ± 55.6 | 45.6 ± 17.4 | 15.0 ± 5.3 | 161.5 ± 82.8 | 19.0 ± 10.2 | ||
| No (N=43) | 59.2 ± 26.0 | 200.3 ± 49.7 | 39.7 ± 15.7 | 14.2 ± 6.1 | 149.2 ± 79.4 | 18.8 ± 17.6 | ||
| t=-1.94, p=0.06 | t=-1.52, p=0.13 | t=-1.36, p=0.18 | t=-0.53, p=0.60 | t=-0.57, p=0.57 | t=-0.04, p=0.97 | |||
| Albuminuria | ||||||||
| Yes (N=20) | 74.9 ± 39.2 | 226.1 ± 58.6 | 45.4 ± 18.7 | 16.1 ± 7.1 | 161.0 ± 90.3 | 21.2 ± 16.6 | ||
| No (N= 45) | 59.7 ± 25.5 | 198.7 ± 47.5 | 40.1 ± 15.1 | 13.8 ± 5.0 | 150.1 ± 75.7 | 17.8 ± 14.7 | ||
| t=-1.87, p=0.07 | t=-1.96, p=0.06 | t=-1.22, p=0.23 | t=-1.49, p=0.14 | t=-0.50, p=0.62 | t=-0.82, p=0.42 | |||
| Neuropathy | ||||||||
| Yes (N=22) | 63.7 ± 26.0 | 200.4 ± 45.3 | 36.3 ± 15.8 | 13.2 ± 6.9 | 116.8 ± 57.9 | 17.7 ± 16.9 | ||
| No (N=43) | 64.7 ± 33.3 | 210.3 ± 55.5 | 44.6 ± 16.2 | 15.1 ± 5.1 | 173.0 ± 84.0 | 19.5 ± 14.6 | ||
| t=0.13, p=0.90 | t=0.70, p=0.48 | t=1.95, p=0.06 | t=1.26, p=0.21 | t=2.74, p=0.008* | t=0.87, p=0.66 | |||
*significant at level of p = 0.05. IFN-γ: IFN-gamma; IL-1β: Interleukin-1 beta; IL-6: Interleukin-6; IL-8: Interleukin-8; ANGPTL-4: Angiopoietin-like protein 4
T2DM is considered closely linked to an obesity-related chronic low-grade inflammatory state, characterised by elevated circulating levels of inflammatory markers which are implicated in the pathophysiology and progression of the disease [4,24]. The findings of the present study reveal that T2DMY patients have significantly higher circulating fasting levels of E-selectin, IFN-γ, IL-1β, IL- 6, ANGPTL-4 and IL-8, compared to healthy subjects without diabetes, even after age adjustment. Moreover, this increase in circulating inflammatory markers in T2DMY patients appears to be linked to central body fat distribution, poor glycaemic control and microvascular complications.
The relationship between elevated inflammatory markers and chronic cardio-metabolic diseases has been clearly established in large epidemiological studies in older adults [25]. Previous studies have shown that circulating inflammatory markers are tightly regulated at very low levels in young individuals [26]. Our results show that patients who develop T2DM at a young age have significantly higher levels of key inflammatory markers (E-selectin, IFN-γ, IL-1β, IL-6, ANGPTL-4 and IL-8) compared to those without diabetes, suggesting a potential link between T2DMY and enhanced pro-inflammatory pathways.
Reinehr et al. [20] have reported significantly higher concentrations of high-sensitivity C-reactive protein, tumour necrosis factor-α (TNF-α) and IL-1β, but not IFN-γ, in obese adolescents with T2DM compared with obese controls without T2DM. The T2DMY patients in our cohort were predominantly obese and had poor overall glycaemic control. Therefore, it is plausible that the noted upregulation of the measured inflammatory markers in the T2DMY group could be linked to chronic hyperglycaemia and obesity-induced insulin resistance. Indeed, these factors are recognised as triggers for a cascade of reactions leading to β-cell and endothelial dysfunction [27]. Obesity is characterised by higher circulating concentrations of inflammatory biomarkers, especially IL-6 and IL-1β, which are considered to play a significant role in the development of insulin resistance [28-30]. Previous studies indicate that IL-1β plays a fundamental role in the initiation and amplification of β-cell inflammation and dysfunction [31], under the influence of other cytokines originating from pro-inflammatory processes in the adipose tissue, as a consequence of obesity [8]. In our T2DMY cohort high circulating levels of IL-6 were positively correlated with high fat mass and waist-to-hip ratio, suggesting that obesity (particularly central) plays a role in the modulation of this cytokine. These findings are consistent with other studies reporting that T2DM patients with obesity exhibit higher IL-6 levels compared to obese controls [32]. Furthermore, IL-6 is recognised as a regulator of glucose metabolism in healthy individuals, whereas in obese individuals it may contribute to the pathogenesis of T2DM by impairing insulin signalling and β-cell function [3,33].
Interestingly, angiopoietin-like proteins are also expressed in adipocytes and obesity-induced inflammation may contribute to their upregulation [34]. Particularly, ANGPTL-4 is involved in regulating lipid and energy homeostasis, and is predominantly expressed in adipose tissue and the liver [14]. Indeed, existing data indicate that ANGPTL-4 levels are increased in patients with T2DM or metabolic syndrome compared to healthy controls [12]. This agrees with our results and could be associated to higher triglycerides levels in the T2DMY patients compared to controls, as ANGPTL-4 is a regulator of plasma triglyceride levels through inhibition of lipoprotein lipase activity [35]. Moreover, genetic inactivation of ANGPTL-4 appears to be associated with improved glucose homeostasis and reduced T2DM risk [36].
Recent research has also focused on the role of IL-8 in diabetes, with a cross-sectional study in T2DM patients (age: 49-67 years) showing significantly higher serum IL-8 levels than non-diabetic subjects, and an IL-8 association with worse pro-inflammatory, metabolic and lipid profiles [11]. Notably, IL-8 has been found elevated in both T1DM and T2DM patients with poor glycaemic control [37]. IL-8 expression from visceral adipose tissue of T2DM patients has also been shown to be significantly higher compared to subjects without diabetes, emphasising the relationship between dysregulated adipose tissue in obesity and circulating markers of inflammation [38].
Furthermore, in accord with our results, Matsumoto et al. [39] have found that circulating soluble E-selectin levels are significantly elevated in association to insulin resistance and total body fat, postulating that obesity may induce endothelial activation and subsequently increase serum levels of this key endothelial adhesion molecule. Of note, it is considered that obesity could increase soluble E-selectin levels partly through a decrease in adiponectin [39] and partly through stimulation by exposure to inflammatory cytokines linked to insulin resistance [16]. Moreover, chronic hyperglycaemia causes accelerated formation of advanced glycation end-products (AGE) and mitochondrial overproduction of reactive oxygen species (ROS) [15]. The subsequent toxic and oxidative stress contributes to dysfunction of the vascular endothelium, which promotes micro- and macrovascular complications [15].
In our study, we focussed particularly on the potential relationships between inflammatory markers and diabetes-related microvascular complications (albuminuria, retinopathy and peripheral neuropathy), as the burden of microvascular complications appears to be high amongst adolescents and young patients with T2DM [40]. In the present study, we found higher levels of circulating E-selectin in patients with combined albuminuria and retinopathy. Soluble E-selectin represents an important biomarker of endothelium dysfunction [41], since it is an endothelial adhesion molecule which is not constitutively expressed, but is only expressed and released from endothelial cells activated by cytokines. Indeed, IL-1β is among those pro-inflammatory factors contributing to endothelial dysfunction through stimulation of E-selectin expression [41]. Data also suggest that IL-1β may play a role in the progression of diabetic nephropathy through inter-glomerular hemodynamic impairment [42]. IL-1β production is influenced by IFN-γ, which also stimulates the expression of cytokine receptors in mesangial cells [43] and acts as an indirect inducer of angiogenesis through the activation of endothelial growth factors, compromising vasoregulation and resulting in increased endothelial permeability [44]. Finally, previous studies have shown that IL-6 is implicated in the pathogenesis of diabetic nephropathy and is associated with albumin excretion and renal hypertrophy [45].
Notably, the link between metabolic dysregulation and chronic inflammation is also considered to be implicated in increased CVD morbidity in T2DM patients [46]. Our findings of higher circulating inflammatory markers in T2DMY patients agrees with this, highlighting the need for additional prospective studies examining the links between these biomarkers and long-term CVD risk. In a study by Li et al. [18] involving 98 young subjects with newlydiagnosed T2DM (aged 10-24 years), low-grade inflammation was associated with a more adverse CVD risk profile and stiffer arteries compared to their adolescent counterparts without diabetes. Furthermore, the TODAY study group [19] has also demonstrated that cardio-metabolic risk is affected by systemic inflammation in a cohort of adolescents (age: 10-17 years) with obesity and T2DM, especially among those receiving insulin treatment. In addition, the atherogenic and inflammatory risk profile documented in the TODAY cohort deteriorated over time, despite intensive intervention, suggesting increased CVD prevalence in the third and fourth decades of life [47,48].
Study limitations
Limitations of the present study include the relatively small sample size, the lack of a BMI-matched healthy control group and the lack of an older T2DM group for additional comparisons. Furthermore, T2DMY adults with established CVD were excluded from the present study. Finally, as this is a cross-sectional observational pilot study potential causal relationship cannot be established based on the reported associations. Clearly, these findings need to be verified in studies involving a larger sample size. However, the present study offers novel data to explore the circulating inflammatory profile in T2DMY patients, thus providing the basis for further prospective and interventional studies into this field.
In conclusion, the present study demonstrates that young adults diagnosed with T2DM under the age of 40 years exhibit significantly higher circulating levels of key inflammatory markers (E-selectin, IFN-γ, IL-1 β, IL-6, ANGPTL-4 and IL-8) compared to healthy controls. In addition, we observed a positive association between selected pro-inflammatory biomarkers, central obesity and microvascular complications particularly E-selectin which appears to be associated with microvascular complications in patients with T2DMY. Future prospective studies are needed to further elucidate the exact role of these inflammatory markers in the development and progression of diabetes in young T2DM patients.
Citation: Lascar N, Afzal I, Nevill AM, Shabir K, Kyrou I, Brown JE, et al. (2020) Increased Circulating Levels of Inflammatory Markers in A Cohort of Adults with Youth-Onset Type 2 Diabetes Mellitus. J Diab Metab 11: 853. doi: 10.35248/2155-6156.20.11.853
Received: 21-Jul-2020 Published: 26-Aug-2020, DOI: 10.35248/2155-6156.20.11.853
Copyright: © 2020 Lascar N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.