Review Article - (2013) Volume 0, Issue 0
Modernization of our lifestyle and absurd eating habits has taken its toll on the human health. People are getting affected by diabetes and this number is on a gradual rise. Diabetes is considered among one of the most deadly disease with a wide variety of symptoms. While diabetes is detected by the blood glucose level and it may only be recorded when the symptoms of this disease starts surfacing and gets beyond any control. Interestingly, a link of this disease has been established with the bacterial infection and directly as well as indirectly with the immune response, thus causing the lipid peroxidation in an indirect way. Lipid peroxidation has also been reported in the patients suffering from diabetes. In this review, we try to establish a critical link between the lipid peroxidation and diabetes. This will help in considering lipid peroxidation as an early marker for diabetes. It will improve the present detection system and thus will help to control this deadly disease.
Keywords: Lipid peroxidation; Diabetes; Microorganisms; Immunity; Reactive oxygen species; Respiratory burst
Diabetes is a chronic disease with serious metabolic disturbances in carbohydrate, protein and fat metabolism arising due to insulin deficiency or insulin action. According to WHO reports, diabetes is among the most serious and widely spread disease. It is mostly spread in the low to middle income countries and has affected approximately 347 million people [1]. It is not only common in older people, but is now frequently occurring in younger generations as well. The reason for this might be linked to the changed life style and food. People are getting obese day by day and are suffering from a variety of diseases, where diabetes is also one of these. The microbial infections in humans are also on a continuous rise, the reason being the development of new resistant strains due to the increased use of antimicrobial chemicals.
A variety of symptoms are linked to this disease, with the most common being thirst, polyurea, blurring of vision and weight loss. On advanced stages, as a result of hyperosmolar state, serious symptoms such as stupor and coma may develop, which if left untreated may lead to death [2]. Beside these primary symptoms, some serious complications may be involved if the glucose level of the blood is not controlled in time. Some of the complications are cardiovascular disease including heart attack, severe neuropathy, retinopathy, nephropathy, osteoporosis and foot damage. Some rare cases of a linkage between diabetes and cancer have also been reported [3,4].
Immune system of an individual works in a very well organized manner for the sustenance and disease free life. The cells involved in this mechanism communicate with each other for increasing the efficiency of this process [5]. Unfortunately, the immune system can be overprotective in some cases, leading to an increased emission of the oxidative molecules. This increased release of free radicals for neutralizing the stress in an individual is the key cause of the oxidative stress. These free radicals create the diabetic complications such as retinopathy, neuropathy etc. and is also a leading cause of lipid peroxidation [6]. This non enzymatic lipid peroxidation resulting from reactive oxygen species has been encountered in the diabetic patients [7]. A breakthrough discovery by Fei and Zhao [8], has recently proved the link between microbial infections and diabetes. Both the microbial infections and diabetes [type 2] in individual as well as in combination are the reason for the activation of immune response [8-11]. In this review, we thrive to provide a link between the lipid peroxidation and the diabetes, increasing an insight in the mechanisms linked to it. This will help bring an improvement in the early detection of diabetes and thus an attempt to prevent it.
Diabetes represented by a universal blue circle is a disease in which a person has an elevated level of blood sugar level, that may be triggered either due to an insufficient amount of insulin produced by pancreas or due to the non-responsiveness of the cells against insulin. This disease was found to be present in humans even in 200 A.D. as described by a Greek physician Arateus, who gave this disease its name. Although, it was present since so many years, still it is not considered a threat until the 20th century. This disease is now considered as a serious threat, because millions of people have died due to this. As stated by WHO, 347 million people worldwide are reported to have different types of diabetes and there is a gradual increase in the number of patients. It was observed that 2.8% world population was suffering from diabetes in 2000, which increased to 6.8% in 2010 and is expected to further rise to 7.7% in 2030 [12,13].
There are primarily three types of diabetes depending upon its basic cause, type 1 diabetes, type 2 diabetes and type 3 diabetes. The differentiation between the different types of diabetes was first observed by Dupertius, which was later verified and confirmed by Lister and Cudworth [14,15]. Type 1 diabetes is also frequently referred as insulin-dependent, juvenile or childhood diabetes. It is characterized by inefficient or no insulin production by the pancreas. It is one of the most common type of diabetes in children and is divided into two types viz. Type 1A and Type 1B diabetes. While type 1A is immune mediated diabetes, where the immune cells are responsible for the destruction of the islet of langerhans β cells [responsible for insulin production] present in pancreas. The type 1B diabetes on the other hand is caused by the inefficient production of insulin by the islet of langerhans β cells. The cause for this diabetes is still unknown and is usually cured by administration of external insulin [16].
Type 2 diabetes is most common and constitutes a total of 90% of all the cases. It is usually caused by the insulin resistance produced in the body. Although, the pancreas produces insulin efficiently the body is unable to recognize and utilize it, leading to the increase in the glucose concentration in the blood. It is linked with several factors such as lifestyle, genetic factors, obesity and even lack of sleep [17-20]. Type 3 diabetes often referred to as gestational diabetes, occurs in pregnant women and is not very common. It resembles type 2 diabetes due to its insulin dependent characteristics. Most of the women affected by this disease are often affected by the type 2 diabetes during their later part of life.
The main question now arising is regarding the possible cause of this disease. The causes of the diabetes are generally dependent on the different types of diabetes and may also depend on the age, sex and physique of the person. Diabetes is generally caused by the controllable factors related to lifestyle, eating habits and insufficient sleep [17,19,20]. But it may also be linked to certain uncontrollable factors such as the false action of immune system and the microbial infection.
The research by Fei and Zhao [8] has given strong ground to the earlier hypothesized link between bacteria and obesity. They have performed the clinical trial on the volunteers suffering from obesity, hypertension and diabetes. It was observed that these volunteers were containing Enterobacter spp. in their gut, which constituted about 35% of the gut microflora. To establish the link between the bacteria and diabetes, the volunteers were fed on a whole grains, traditional Chinese medicinal foods and prebiotics [WTP] diet. Interestingly, it was observed that after 23 weeks of continuous diet, these volunteers had undetectable concentration of the bacteria. As a result, the volunteers lost around 50 kgs of their weight and also were free from diabetes. This clearly showed that the bacterial infection of the endotoxin producing Enterobacter spp. may also be a reason for the diabetes among the patients. The rise in the number of diabetic patients suffering from obesity may also be associated with the bacteria.
Diabetes is also caused by the false immune response of the body, in other words, diabetes is also caused by the immune response against the self cells and the self biomolecules. Immune response of the body is directly linked with all the three types of the diabetes. The islet of langerhans β cells are destroyed by the immune cells causing type 1 diabetes. It leads to either insufficient amount of insulin production or even no insulin production depending on the damage done by the immune system (Figure 1). Although the reason for this is not clear, but many researchers linked type 1 diabetes with T cells [16]. Later studies were able to prove the role of autoreactive CD8+ T cells in causing the type 1 diabetes and both humoral and cell mediated autoimmunity were found to be the causative agents of this disease [21,22]. Similarly, type 2 and type 3 diabetes is also linked to the immune response. This diabetes being the most common, is highly studied by the researchers. It is commonly known as the insulin independent diabetes, as there is no effect of insulin on blood glucose level (Figure 1). Many hypotheses have been proposed regarding the inflammatory cause of diabetes. It was also hypothesized that the diabetes may be an outcome of the nonspecific response of immune system. The association of interleukin-6 [IL-6] and C-reactive proteins [CRP] with diabetes has been confirmed by many researchers as it was found present in the patients [23]. In general, the acute phase-markers were found to be interlinked with this disease. Review by Pickup [23] has clearly explained the effect of nutrition on the quantity of CRP and IL-6. An increase in the fat content in the meal leads to an increase in CRP and IL-6, thus increasing the risk of diabetes. With the increase of obesity in a person, the adipose tissue becomes saturated with fat. These adipocytes starts secreting low level of tumour necrosis factor-α [TNF-α], which in turn stimulates preadipocytes to the production of monocyte chemoatractant protein-1 [MCP-1]. This is responsible for attracting the macrophages towards the adipose tissues. The increased lipid content of the adipose tissue also triggers the oxidative damage and thus the crowding of the macrophages [24]. The researchers found F4/80 antigen on the lipid saturated adipose cells. It was found that when the bone marrow of mice was lethally irradiated and fed on the fat rich diet, F4/80 antigen was found absent. This antigen was also linked with the response of macrophages to the fat cells. The continual action of macrophages on the fat cells and the increase in the size of the adipose tissues with the presence of macrophages, leads to the production of TNF-α, nitric oxide synthase [iNOS] and nitric oxide [NO] by the adipose cells. These factors contribute to the resistance and thus a decreased insulin dependent glucose uptake [25].
Respiratory burst is a remarkable property of the neutrophils, macrophages, B cells and other phagocytic cells [26,27]. These cells after activation increase their oxygen uptake, which may raise upto 50 folds [28]. This increased oxygen uptake is then followed by the breakdown of this oxygen by the enzyme respiratory burst oxidase as shown below:
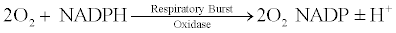
The superoxide radical formed from the above reaction is responsible for a variety of reactions, forming products such as oxidized halogens, oxidizing radicals and singlet oxygen [29]. This reaction is particularly important at the time of infections. These are known to be highly lethal for the micro-organisms and are found to cause damage to the microbial nucleotides, redox enzymes, proteins and the most important among all damage is the genetic mutation observed after the action of phagocytes [28,30-32].
The superoxide radicals and other reactive oxygen species formed from the above reactions are not only responsible for protection from micro-organisms, but they also deal damage to the self cells. These have also been found in patients suffering from diabetes. Researchers have stressed on the critically increased respiratory burst in the polymorphonuclear (PMN) leukocytes [in both resting as well as stimulated state] in the patients suffering from diabetes as compared to the non-diabetic control [33]. The main reason may be the increased obesity, which leads to the production of factors such as TNF-α by the adipose tissues. This TNF-α is also known to regulate the NADPH oxidase activity, which in turn is responsible for the superoxide generation. So, diabetes is indirectly caused by the activation of superoxides in neutrophils [34].
Lipid peroxide is a very important compound formed by the chain reaction. Here, the non radical lipids are converted to radicals by species such as O.2, OH., NO. and other reactive oxygen species (ROS). The lipids have hydrogen group attached to them, and this hydrogen is having a proton and an electron. When the free radical removes this hydrogen atom, it leaves behind an unpaired electron in the lipids [35]. This in turn leads to a chain reaction, thus reacting with other biomolecules (Figure 2) [35,36]. The reaction has been mentioned below:
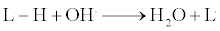
This lipid peroxidation results in the chain reaction and damages the various other molecules, finally leading to the cell damage [37]. Upon lipid peroxidation, a variety of products are formed depending on the type of lipids and the location of the electron. These products are malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), lipid hydroperoxide (LOOH), isoprostanes, conjugated dienes, lipid-DNA adduct, lipid-protein adduct, lipofuscin pigments, exhaled gases [38].
Lipid peroxidation has been reported to occur by both infection as well as inflammation. It has been reported by Khovidhunkit et al. [39] that during the onset of inflammation or infection there is a clear increase in the level of serum triglyceride level. An increase in the high density lipids (HDL) level is usually observed along with the proteins associated with it. Both the infection and inflammation are responsible for the change in the composition and functions of lipoproteins. It mainly includes the changes in the sphingolipid concentrations, decrease in the reverse cholesterol transport and an increase in the lipid oxidation.
It has been hypothesized that microorganisms activate the enzyme phospholipase A2 following an attack on the cell. This enzyme in turn hydrolyzes the sn-2 position of the phospholipid and produces fatty acids and lipophilic substances [40]. The microorganism causes changes in the cell membrane and triggers the activation of the enzyme which generates free fatty acids. These free fatty acids might play an important role in modifying the pH. The membrane change in addition may also activate the cyclo-adenosine-monophosphate, which in presence of Ca2+ ions activates kinases. Which in turn might activate the lytic enzymes such as lipoxygenases (LOX)? The LOX upon activation undergoes the oxidation of the Fe2+ [present in the active center] to Fe3+. This oxidation requires the removal of a hydrogen atom from the CH2 group, leading to the formation of hydrogen radical. The lipid radical reacts with oxygen, leading to the formation of peroxyl radical (LOO.). This radical then takes an electron from Fe2+ and is converted into peroxyl anion [LOO-]. The peroxyl anion finally combines with the generated proton to form LOOH [40]. Since the bacteria responsible for diabetes and obesity i.e. Enterobacter spp. is an endotoxin, which are lipopolysaccharides (LPS). These LPS are the core structure of the outer membrane of the bacteria and are generally removed during the cell lysis. The activity of the immune system on this lipid may in turn lead to either the lipid peroxidation of the self lipid molecules by the generation of reactive oxygen species or may lead to the development of resistance among the immune cells to the lipid and thus leading to the lipid peroxidation by the free radicals [41].
It is also evident that the diseases that lead to the activation of inflammatory processes are usually linked with the walls of the affected tissue i.e. both the infection and inflammation leads to the change in the cell membrane [40]. The lipid peroxidation during diabetes is generally caused by both the enzymatic methods as well as the nonenzymatic methods. It has already been mentioned in this section that the activation of the enzyme phospolipase A2 is linked with the change in the conformation of the cell membrane. Diabetes is linked with the high blood glucose level and the high lipid content of the adipose tissues during obesity. This leads to the increase in the size of adipocytes and thus leading to the generation of phospholipase A2. The activation of phospholipase A2 finally leads to the process of the lipid peroxidation [40]. The non-enzymatic process is usually caused by the mitochondrion. It is well known that mitochondrion is responsible for the transformation of oxygen into water. This involves the conversion of oxygen into superoxide anion by the transfer of an electron. These superoxide radicals are sometimes able to escape and react with water, thus generating HO.2, which is converted to H2O2 and O2. The H2O2 thus formed is a radical and reacts with Fe2+ ion to form an OH. radical. The OH. radical removes an hydrogen atom from CH2 group and initiates the chain reaction leading to the process of lipid peroxidation [40]. The lipid peroxidation is generally linked with the reduced tolerance among the T cells for the self molecules due to the general processes, infections and diseases like diabetes [42].
The concept of the diabetes, ROS and lipid peroxidation has already been stated above, now the link among the three has to be established. Diabetes may occur by either the inefficient or no insulin production, resistance to insulin and by microbial infection. It has also been discussed that the immune system is responsible for direct or indirect role in all the three reasons of diabetes. Since, the immune system is responsible and the immune reaction involves the reactive oxygen species, which in turn causes lipid peroxidation. Diabetic patients have also shown an increased ageing i.e. the processes or the characteristics and symptoms present during ageing [43]. The ageing is generally linked with the lipid peroxidation by ROS and so the diabetic has a role in lipid peroxidation.
Akkus et al. [44], have clearly shown that immune reactions involves ROS, which also causes lipid peroxidation. Although many enzymes/substances are available in the body which have the ability to control these radicals such as vitamin C, superoxide dismutase and glutathione peroxidise etc. But still, many reports have also suggested the defective metabolism of vitamin C along with the altered leukocytes in patients suffering from diabetes [45]. It has also been reported that diabetic patients are shown to possess a lower quantity of α-tocopherol, vitamin C, total glutathione and isoprostance in the plasma [46]. In addition, many researchers have shown an increased MDA level (lipid peroxidation product) in patients suffering from diabetes [47]. Feillet- Coudrey et al. [48] have clearly showed an increased antioxidant activity in experimentally induced diabetes inspite of the increased vitamin E concentration. Finally, Kojda and Harrison [49] have shown that the patients supplied with antioxidant probucol and/or superoxide dismutase showed reduced diabetic complications and thus a clear connection between the two.
The above explanations have clearly shown the presence of lipid peroxidation during diabetes. Although, no single reason the lipid peroxidation could be pinpointed, but still it could be said that the diabetes is caused by several reasons and each reason can directly or indirectly be linked to the immune system. This immune system is somehow defective in diabetes and thus leads to the lipid peroxidation (Figure 3). Since, lipid peroxidation is shown to occur during diabetes, we could easily measure this by a variety of methods as described in table 1 and somehow put an early curb to this deadly and epidemic disease.
| Method Employed | Substance measure | Reference |
|---|---|---|
| TBA Test | Thiobarbituric acid reactive material (TBARM) | Garcia et al. [50]; Groussard et al. [51]; Seljeskog et al. [52] |
| Conjugated dienes | Diene-conjugated structures | Devasagayam et al. [38]; Holley and Cheeseman [53] |
| Fluorescence | Aldehydes | Gomes et al. [54] |
| Spectrophotometry | Fe(III)xylenol orange complex formation | Gay and Gebicki [55] |
| Spectrophotometry | Iodine produced | El-Saadani et al. [56] |
| Mass Spectrophotometry | Cholinergic phospholipids | Brouwers et al. [57] |
| GC-MS | Aldehydes | Kawai et al. [58] |
| Radioimmunoassay method | Prostaglandins | Pepicelli et al. [59] |
| GSHPx | Lipid peroxides | Taysi et al. [60] |
| HPLC | Lipid hydroperoxides | Wood et al. [61] |
| Iodine liberation | Lipid Peroxides | Sano et al. [62] |
| Spin Trapping | Intermediate radicals | Rosen and Rauckman [63] |
Table 1: Different methods for measuring lipids.
In nutshell, we would like to conclude that diabetes is an epidemic that occurs due to a variety of reasons among which the lifestyle and eating habits being the most common. These together have lead to a change in the immune system and lead to a defective respiratory burst, which somehow leads to the lipid peroxidation and thus damages various other cell molecules. Although, many clinical tests are available to detect the presence of this disease, but these tests could only highlight the diabetes after the onset of this disease. After the detection, one could only rely on the precautions and is bound by this disease forever. In the hope to provide the method for the early detection of this disease, we have stressed upon the use of lipid peroxidation as an efficient method. Diabetes by obesity and microbial infection can easily be identified at an early stage and the proper cure can prevent it. Still some research is required to validate this hypothesis and hopefully it will benefit and save millions of people present on the verge of onset of this disease.