Research Paper - (2021) Volume 12, Issue 11
This is an open label randomized control study comparing 2 protocols of mannitol administration in laparoscopic donor nephrectomy: upon adequate exposure of kidney hilum and 15 minutes prior to cross clamping. We observed its effects on the intraoperative mean arterial pressure (MAP) of donor patients, daily urine output, creatinine clearance and the length of hospital stay of graft recipients.
Forty-two pairs of subjects were enrolled after written consent was obtained. Computer-generated randomization assigned the allograft donor-recipient to 21 pairs per treatment arm. Group A was administered mannitol 0.25 g/kg before skin incision and 0.5 g/kg once the kidney hilum was adequately exposed. Group B received mannitol 0.25g/ kg before skin incision and 0.5g/kg 15 minutes prior to cross clamping. All 42 pairs successfully completed the study.
Mannitol, when administered 15 minutes prior to cross clamping, significantly increased urine output in day 1 posttransplantation. The creatinine clearance and length of hospital stay were independent of the timing of mannitol administration.
Acute tubular necrosis; Allograft protection; Kidney transplantation; Mannitol; Urine output post-transplant
Goals in preserving kidney graft during live donor harvest include adequate hydration with crystalloids, a targeted mean arterial pressure (MAP), avoidance of hypotension and adequate urine output [1]. Strong experimental data by van Valenberg and colleagues reported their experience in a randomized study of hydration with mannitol vs hydration without mannitol in cadaveric renal transplants. These results demonstrated a significant reduction in postoperative acute tubular necrosis in patients treated with mannitol [2].
The use of mannitol as a protective agent during renal transplantation stems from its ability to increase renal blood flow, thought to be the result of a release of intrarenal vasodilating prostaglandins and/or atrial natriuretic peptide. A second perceived benefit of mannitol is related to its role as an oxygen free-radical scavenger that may impart some protection to the kidney following reperfusion of the renal allograft [3].
Studies using animal models have indicated that mannitol must be delivered 15 minutes before warm ischemia insult in order to prevent ischemic-induced acute tubular necrosis (ATN). Andrews, et. al. reported the same time-dependent effect of mannitol in humans. Using optical coherence tomography (OCT), their study revealed that patients given mannitol 15 minutes before cross clamping results to faster graft recovery in the first week after transplantation than those treated with mannitol 30 minutes or more before clamping [4]. Current practice in renal transplant anesthesia in National Kidney and Transplant Institute (NKTI) recognizes mannitol as indispensable tool in the protection of kidney graft. The usual protocol is to administer 0.5 mg/kg mannitol once the renal hilum is adequately exposed. This means a 30 minute or more interval from mannitol infusion until renal artery clamping.
While imaging techniques and post-transplant serum creatinine were the outcome indicators of previous studies, we compared present practice versus 15 minutes prior to cross clamping by observing its effect on the intraoperative mean arterial pressure (MAP) of donor, and overall graft outcome such as postoperative mean 24-hour urine output, creatinine clearance and the length of hospital stay.
Investigators of this study aim to influence present practice guidelines in perioperative anesthetic management of kidney transplantation. In the field of future research, we aim to provide contributions for further related studies in the prevention of acute kidney injury resulting to improved overall graft outcome and survival.
Objectives of the Study
This study seeks to compare the timing of mannitol infusion once kidney hilum is adequately exposed versus 15 minutes prior to cross clamping during laparoscopic donor nephrectomy and observe its outcome on graft recipients. Specifically, it aims to describe the demographic and clinical profile of kidney allograft recipients such as age, sex, and body mass index, etiology of renal failure, baseline creatinine clearance, and total ischemia time. It aims to compare the intraoperative MAP of donor patients, the duration from the time of artery clamp to the start of mannitol infusion and the outcome on the allograft recipients such as daily urine output, creatinine clearance, and the length of hospital stay.
Study design
This is an open-label randomized control trial. The patients, anesthesiologists in charge and investigators were aware of the treatment being given.
Population
This study included 42 pairs of kidney allograft donor-recipient patients undergoing elective laparoscopic donor nephrectomy (related or non-related), undergoing elective kidney transplant procedure (living related or living non-related), 18-60 years old, both sexes and those who gave consent. Cadaveric donor transplant recipients and open donor nephrectomy patients were excluded.
Definition of terms
Creatinine clearance using CKD-EPI formula - CKD-EPI formula was developed in 2009 by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). This was recently developed and validated in pooled populations with diverse clinical characteristics. It is a recent formula which was shown to be more accurate, especially for higher GFR values and also provide lower estimates of CKD prevalence and to categorize more appropriately individuals with respect to renal and CVD risk, as compared with the MDRD Study equation [5].
Method sampling
Forty-two pairs of patients were randomized according to treatment arms using Microsoft Excel 2007 randomization function. This was conducted by the investigator. The randomization list assigned patient number to either treatment A or B. A printed copy of the list was handled by the investigator.
Donors were hooked to standard American Society of Anesthesiologists (ASA) monitors such as ECG, pulse oximeter, end tidal carbon dioxide (ETCO2) and non-invasive blood pressure. Crystalloid infusion of up to 20 ml/kg/h was infused. In Group A, 0.25 g/kg (50-100cc) mannitol was administered before skin incision and 0.5g/kg once the hilum was adequately exposed. In Group B, patients received 0.25 g/kg mannitol before skin incision and 0.5g/kg 15 minutes prior to cross clamping. Constant communication with the surgeon was necessary to ensure the correct timing of the infusion. The start of second mannitol infusion and the time of artery clamp as well as the MAP were recorded.
Postoperatively, the mean hourly 24-hour urine output, daily serum creatinine, and the length of hospital stay of allograft recipients were recorded.
Data handling and analysis
Data was recorded on a pre-designed research data collection form. The demographic and biomedical data were encoded using Microsoft Excel 2007. The patients’ baseline characteristics were described and compared using means, percentages and standard deviations.
Analytical statistics was utilized to determine significant differences between two variables. The null hypothesis states that there is no significant difference between the urine output and serum creatinine levels for both groups. To determine statistical difference with the daily urine output, creatinine clearance and the length of hospital stay as the primary endpoints, the Students t-test was utilized. The level of significance to reject the null hypothesis was set at <0.05.
The Repeated Measures ANOVA was utilized as another statistical approach to determine significant differences of the means across time since the study required serial measurements of the mean arterial pressure of the donor patient, the duration from the time of artery clamp to the start of mannitol infusion, the daily urine output, creatinine clearance and the length of hospital stay.
Ethical considerations
The informed consent was explained in a dialect easily understood by the patient and was done prior to patient enrolment. The study was conducted in compliance with the protocol and regulatory requirements. All information remained confidential. The interview schedules were kept in a data storage room so that only the investigator had access of it.
Forty-two pairs of subjects were enrolled after written consent was obtained. Computer-generated randomization was performed to assign the allograft donor-recipient pairs into two groups. All 42 pairs successfully completed the study until the last day with corresponding protocols in each group effectively executed. No adverse events were noted in all of the subjects during or after the surgery (Table 1). Comparison of the demographic and clinical profile of kidney allograft recipients and ischemia time of allograft. Allograft recipients were evenly distributed in terms of demographic and clinical profile and length of ischemia (Figure 1). The top 3 most common causes of CKD among allograft recipients are chronic glomerulonephritis, diabetic kidney disease, and hypertensive nephrosclerosis, respectively (Table 2).
| Variables | Group A (n=21) | Group B (n=21) | p-value |
|---|---|---|---|
| Age in yrs, x (+/-SD) | 42.86 (11.42) | 44.52 (12.82) | 0.66 |
| Sex, n (%) | |||
| Male | 12 (50) | 12 (50) | 0.75 |
| Female | 9 (50) | 9 (50) | 0.75 |
| BMI, x (+/-SD) | 30.92 (31.70) | 24.70 (3.98) | 0.37 |
| ETIOLOGY OF CKD, n (%) | |||
| Hypertensive Nephrosclerosis | 4 (66.6) | 2 (33.3) | 0.66 |
| Diabetic Kidney Disease | 4 (33.33) | 8 (66.67) | 0.3 |
| Chronic Glomerulonephritis | 11 (52.38) | 10 (47.62) | 0.76 |
| Uric Acid Nephropathy | 2 (50) | 2 (50) | 0.75 |
| Focal Segmental Glomerulosclerosis | 1 (100) | 0 (0) | 0.5 |
| NSAID Nephropathy | 1 (100) | 0 (0) | 0.5 |
| Henoch Schonlein Purpura Nephrosclerosis | 0 (0) | 1 (100) | 0.5 |
| Baseline Creatinine Clearance, x (+/-SD) | 5.78 (1.81) | 10.64 (12.61) | 0.08 |
| Total Ischemia Time, x (+/-SD) | 70.24 (21.1) | 63.05 (16.13) | 0.22 |
Table 1: Comparison of the demographic and clinical profile of kidney allograft recipients and ischemia time of allograft.
| Variables | Group A (n=21) | Group B (n=21) | p-value |
|---|---|---|---|
| Intraoperative MAP, x (+/-SD) | 82.01 (11.37) | 83.04 (11.73)) | 0.79 |
| Duration of Artery Clamp to Mannitol Infusion, x (+/-SD)(minutes) | 57.85 (33.42) | 12.71 (13.50) | 0.00* |
| *statistically significant p value at <0.05 | |||
Table 2: Clinical profile of donors.
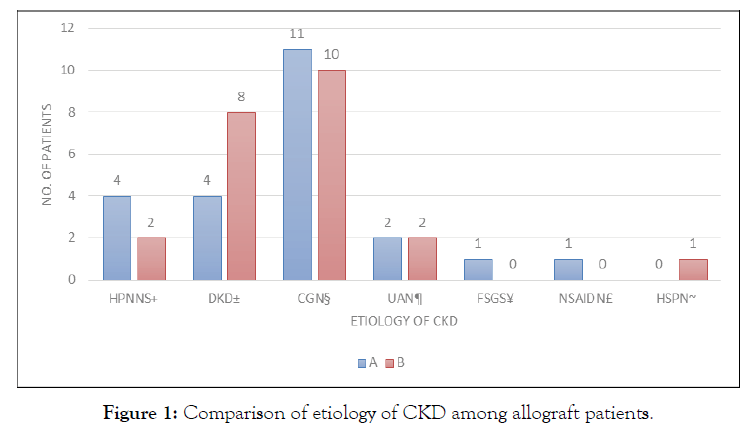
Figure 1: Comparison of etiology of CKD among allograft patients.
Intraoperative MAP was acceptable and similar in both groups. Significant difference in the duration of artery clamp to mannitol infusion established the large disparity between two treatment protocols in terms of initiation of mannitol administration.
Postoperative Outcomes
Urine output
Comparison of the mean 24-hour urine output from the first postoperative day until the last day of kidney allograft recipients (Table 3).
| Post-op Day | Urine Output for Group A, x(+/-SD)(ml) | Urine output for Group B, x(+/-SD)(ml) | Mean Difference | p-value |
|---|---|---|---|---|
| Day 1 | 4,767.81 (2,442.83) | 6,831.00 (3,300.33) | -2,063.19 | 0.02* |
| Day 2 | 5,424.79 (3,343.54) | 5,732.83 (2,196.71) | -308.04 | 0.73 |
| Day 3 | 5,934.81 (2,140.51) | 6,504.52 (2,528.87) | -569.71 | 0.43 |
| Day 4 | 4,614.76 (1,630.85) | 5,931.19 (2,624.36) | -1316.43 | 0.05 |
| Day 5 | 4,077.76 (1,728.91) | 4,695.90 (1,953.08) | -618.14 | 0.28 |
| *statistically significant p value at <0.05 | ||||
Table 3: Comparison of the mean 24-hour urine output from the first postoperative day until the last day of kidney allograft recipients.
Group B patients had more urine output than group A from day 1 to 5, with the significant difference observed at day 1 (Figure 2). Group B has consistently higher urine volume than group A with the largest difference noted on day 1 and day 4. Only day 1 is significantly different (Tables 4&5).
| Day | Creatinine Clearance for Group A, x(+/-SD) | Creatinine Clearance for Group B, x(+/-SD) | Mean Difference | p-value |
|---|---|---|---|---|
| Day 1 | 21.02 (11.96) | 28.96 (19.106) | -7.94 | 0.11 |
| Day 2 | 61.75 (30.79) | 61.30 (29.64) | 0.45 | 0.96 |
| Day 3 | 75.10 (28.96) | 74.29 (26.68) | 0.81 | 0.92 |
| Day 4 | 82.12 (25.35) | 81.59 (20.82 | 0.53 | 0.94 |
| Day 5 | 83.92 (21.80) | 85.24 (17.65) | -1.32 | 0.82 |
Table 4: Comparison of the creatinine clearance from the first postoperative day until the last day of kidney allograft recipients.
| Variables | Group A (n=21) | Group B (n=21) | p-value |
|---|---|---|---|
| No.days of Hospital Stay, x (+/-SD) | 6.81 (1.07) | 7.47 (1.36) | 0.08 |
Table 5: Comparison of the length of hospital stay of kidney allograft recipients.
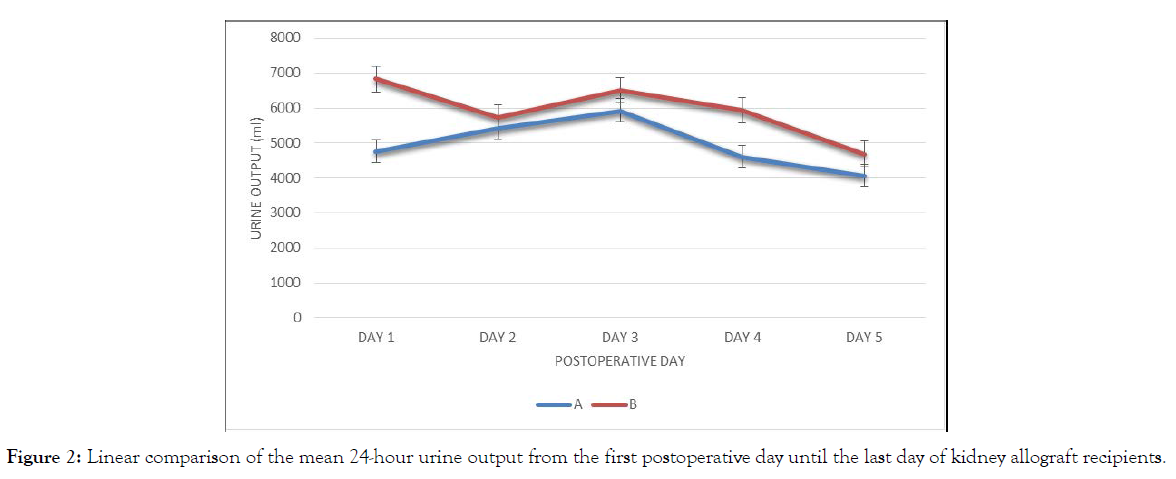
Figure 2: Linear comparison of the mean 24-hour urine output from the first postoperative day until the last day of kidney allograft recipients.
Creatinine clearance
There was no significant difference in the creatinine clearance between two groups from day 1 to day 5. Both groups are parallel in trend, increasing from day 1 to day 5. The largest difference between groups is seen on day 1 (Figure 3).
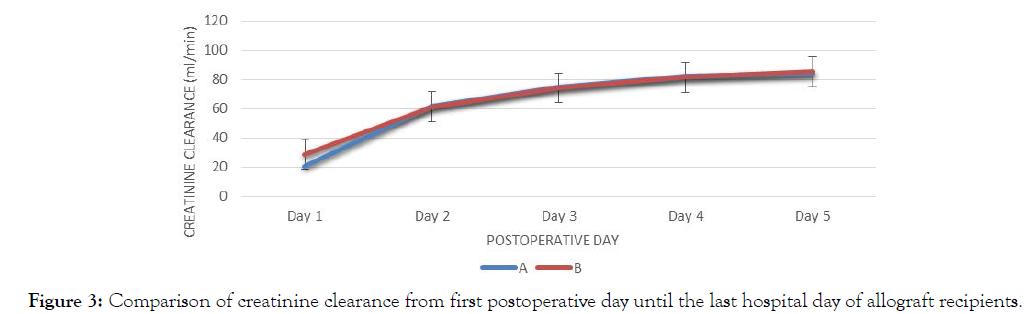
Figure 3: Comparison of creatinine clearance from first postoperative day until the last hospital day of allograft recipients.
Hospital stay
There was no significant difference in the length of hospital stay between the two groups.
We observed consistently higher volumes of urine in patients under group B than in group A, with the significant difference in day 1 post-surgery. A large urine volume is recognized as a good indicator of early graft function, and represents the first sign of progressive recovery, before any decrease in serum creatinine or blood urea nitrogen (BUN) [6-8].
Albeit higher in urine volume than group A, group B exhibits an intermittent change after day 1. Theoretically, interplay of factors in the management of patients may have influenced the result [9]. Pre-transplant recipient factors include immunosuppression, preexisting cardiovascular status, fluids and electrolytes status, and pre-operative dialysis. Intraoperative factors may include anesthetic effects on cardiac output, presence or absence of vasopressors and ischemia time. Postoperative factors include degree of hydration and hemodynamics of patients contributing to fluid balance. In addition, glomerular filtration of donor allograft may also be an additional source of bias.
An understanding in the allograft recipient’s residual urine before surgery is important in interpreting post-transplant urine volume. A comparison of the baseline creatinine clearance in the two groups was done to eliminate potential bias. We used creatinine clearance using CKD-EPI which estimates glomerular filtration rate from serum creatinine while considering other variables such as age, gender and race.
When mannitol is given 15 minutes or less before artery clamp, the renal damage from periods of ischemia may be effectively reversed as opposed to when it is given longer. Histopathologic reports described by Andrews, et. al. showed an increase in tubular lumen from reduction in cell swelling of proximal convoluted tubules when mannitol was given at the 15 minute time interval [4].
Donor Mean Arterial Pressure
Maintaining adequate MAP while study intervention is being given will reflect effective protection of the kidney allograft from acute kidney injury. A MAP of 80 or more assures adequate renal perfusion pressures and reflects reduced ischemia risk for the allograft. With both treatments arms having similarly adequate mean MAP during and after mannitol was infused, perfusion pressures were maintained in the two groups. Such data suggest safety on the patients and subsequently lesser risk of contributory factor for ATN that might confound the postoperative outcome assessment of the study.
Creatinine clearance and length of hospital stay were not affected by the timing of mannitol infusion. Interplay of factors in the perioperative period may influence the outcome of these parameters. Other parameters not included in this study such creatinine trend and blood urea nitrogen (BUN) levels may also have been useful predictors for graft function.
Mannitol, when administered 15 minutes prior to ischemic insults, can increase urine production in day 1 post-transplant. The creatinine clearance and length of hospital are independent on the timing of mannitol administration.
This study emphasized the importance of the correct timing of administering mannitol during graft nephrectomy in order to achieve maximum organ recovery from ischemic insults. We therefore recommend the following:
1. We believe a change in the protocol in perioperative anesthesia practice guidelines is necessary in order to help improve renal allograft survival, stressing on the 15 minute time interval from mannitol to artery clamp.
2. Further study on the effect of mannitol administration during graft nephrectomies with emphasis on long term allograft survival.
3. Further study on parallel research, to include measurements not included in this study, pre-existing cardiovascular status, dialysis status, vasopressor use, glomerular filtration of allograft, serum creatinine and blood urea nitrogen levels.
4. A higher patient population in further studies to yield more conclusive results.
The primary investigator wish to recognize the following individuals who contributed to the success of this study:
1. Dr. Rojim Sorrossa, statistician and a dear friend who has always been reliable and supportive despite our geographical differences.
2. My colleagues, Drs. Elaine Tiblani, Vanessa Adorador, John Paul Urian, and members of the anesthesia team who efficiently executed the treatment protocols.
3. Dr. Jennifer Macaraig, mentor and co-investigator, whose ideas greatly contributed to the success of this study.
4. Most of all, the Almighty Creator, to whom the success of this endeavor, I offer.
Citation: Plarisan MAC, Macaraig JA (2021) Mannitol Infusion in Laparoscopic Donor Nephrectomy. J Diabetes Metab. 12:905. doi: 10.35248/2252-5211.21.12.905
Received: 09-Nov-2021 Published: 30-Nov-2021
Copyright: © 2021 Plarisan MAC, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.