Research Article - (2021) Volume 12, Issue 11
Objective: This study aimed to evaluate the effect of adding vildagliptin to metformin therapy on major CV risk parameters in newly diagnosed patients with type 2 diabetes mellitus (T2DM).
Methods: Forty three eligible patients were prospectively randomized to receive combined vildagliptin/metformin therapy or metformin alone. Anthropometric measurements, blood pressure (BP), glycated hemoglobin (HbA1c), lipid profile, plasminogen activator inhibitor-1 (PAI-1), high sensitivity C-reactive protein (hs-CRP), and total antioxidant capacity (TAC) were assessed at baseline and after 12 weeks.
Results: Forty patients completed the study (20 in each group). At baseline, no significant differences were observed between groups in all studied parameters. After 12 weeks, combined vildagliptin/metformin showed significant reductions in HbA1c (Δ change: -2.68 ± 2.24 versus -1.37 ± 1.8%, P = 0.043, respectively), systolic BP (Δ change: -12.5 ± 13.03 versus -3.75 ± 11.57 mmHg, p= 0.012, respectively), diastolic BP (Δ change: -10.25 ± 9.39 versus -2.5 ± 9.39 mmHg, p= 0.009, respectively), triglycerides (Δ change: -9.7 ± 18.48 versus 10.35 ± 27.36 mg/dl, p= 0.037, respectively), and PAI-1(Δ change: -7.93 ± 17.11 versus 3.9 ± 19.39 ng/ml, p= 0.048, respectively) as compared to metformin monotherapy. No significant differences were observed between both groups regarding their effects on other studied parameters.
Conclusion: Adding vildagliptin to metformin resulted in a decrease in PAI-1, systolic and diastolic BP, TGs, and HbA1c with no significant changes in hs-CRP, TAC, and other lipid markers.
Vildagliptin; Metformin; Cardiovascular risk; Type 2 diabetes mellitus.
Abbreviations: BMI: body mass index; ACE: angiotensin converting enzyme; ARBs: angiotensin receptor blockers; CCBs: calcium channel blockers. HbA1c: glycated hemoglobin A1c; hs- CRP: high-sensitivity C-reactive protein; TC: total cholesterol; TGs: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; PAI-1: plasminogen activator inhibitor-1; TAC: total antioxidant capacity; SBP: systolic blood pressure; DBP: diastolic blood pressure.
The global prevalence of type 2 diabetes mellitus (T2DM) is increasing rapidly with cardiovascular (CV) complications being the main cause of death and disability in affected patients [1]. Hyperglycemia promotes a number of metabolic changes accompanied by the pathogenesis and progression of atherosclerosis and vascular damage. It is associated with the generation of reactive oxygen species (ROS) with subsequent decreased antioxidant defenses, promoting inflammatory state, platelet hyperactivity, and endothelial dysfunction. Moreover, CV risk factors of hypertension and atherogenic dyslipidemia are common among patients with T2DM [2].
Several inflammatory biomarkers have been demonstrated to be increased consequently to the hyperglycemic state [3]. From those is high-sensitivity C-reactive protein (hs-CRP) which has been recognized as an independent inflammatory predictor for CV events and risk assessment. It exerts pro-atherogenic effects implicated in the pathogenesis of atherosclerosis and endothelial dysfunction [4]. Chronic hyperglycemia also alters the coagulation process and leads to higher expression of plasminogen activator inhibitor-1 (PAI-1), an important regulatory element in fibrinolysis, with subsequent elevating the risk of atherothrombotic events [5,6]. All these metabolic features have been considered as the major CV risk factors which are clustering together leading finally to the diabetic vascular complications [2].
Metformin is strongly recommended as the first line therapy, if tolerated and not contraindicated, added to lifestyle modification for managing hyperglycemia in T2DM. For more glycemic control, stepwise addition strategy of other antihyperglycemic agents to metformin is generally accepted and preferred than initial combined metformin based therapy. Starting with the combination therapy may be considered in higher levels of basal glycemia [7]. Previously, metformin was shown to exert protective effects on several metabolic parameters and demonstrated CV benefits [8, 9].
Vildagliptin is one of the dipeptidyl peptidase-4 (DPP-4) inhibitors used for the management of T2DM. It inhibits the DPP-4 enzyme which rapidly degrades both incretin hormones: glucagonlike peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Active GLP-1 and GIP control glucose hemostasis mainly through a glucose-dependent manner by acting on different sites of action [10]. Accumulative evidence reported possible beneficial effects of GLP-1 on the CV system. Moreover, beyond GLP-1, there are other multiple substrates for DPP-4 enzyme involved in regulating vascular function, inflammation, cell migration, and cell differentiation [11]. These findings may suggest the anti‐atherogenic and anti‐inflammatory properties of incretin based therapies illustrated in animal experimental studies [12]. However, these favorable effects have not been well studied in clinical setting especially for vildagliptin. Therefore, this study was designed to evaluate the effect of adding vildagliptin to metformin versus metformin monotherapy as initial therapies for newly diagnosed patients with T2DM focusing on the effect of vildagliptin on major CV risk markers.
Study design and patients
This was a 12-week prospective randomized open-label interventional study. It was conducted in the Internal Medicine Outpatient Endocrinology Clinic, Tanta University Hospitals, Tanta, Egypt. The ethical approval of the study was obtained from the National Research Ethics Committee of Tanta University. All participants signed an informed written consent prior to inclusion in the study. Inclusion criteria were newly diagnosed male and female patients with T2DM according to the American Diabetes Association (ADA) diagnostic criteria of diabetes who aged ≥18 years with risk for developing atherosclerotic CV disease (ASCVD) based on traditional risk factors. Exclusion criteria were patients with renal impairment [estimated glomerular filtration rate (eGFR)≤ 60 ml/min/1.73 m2], known hepatic cell failure, decompensated heart requiring pharmacologic management, active malignancy on clinical bases, history of ASCVD, planned coronary or surgical interventions, known hypersensitivity to either of the study drug components, chronic inflammation, trauma or infection on clinical bases, and any of study treatment labeled contraindications. Pregnant women, those who were breastfeeding, and women using oral contraceptives were also excluded.
The study was initiated with a four-week titration period of metformin hydrochloride prior to randomization. Forty three eligible patients tolerated the maximum metformin dose of 2000 mg daily after this titration period were randomized into two groups: group 1, 21 patients received metformin hydrochloride 1000 mg twice daily (Glucophage®, Minapharm Company, Egypt, under licence of Merck Santé Company, France) and group 2, 22 patients received combined therapy of vildagliptin 50 mg plus metformin 1000 mg twice daily (Vildagluse plus®; vildagliptin 50 mg/ metformin hydrochloride 1000 mg, Inspire Pharma Company, Egypt) for 12 weeks. By the end of study, only 40 patients completed the study (20 patients in each group) ) as one patient was excluded from the metformin group due to dizziness and back pain while two other patients were missed in the combination group.
Follow up visits
All participants were monitored every two weeks. Lifestyle management with all its aspects were offered to all patients as recommended by the ADA at baseline and reinforced in each visit in both groups. All adverse events including hypoglycemia which is defined as blood glucose ˂ 70 mg/dl were reported. Both symptomatic and asymptomatic hypoglycemic events were included. Baseline co-medications were advised to be continued with the same doses and frequencies during the study period.
Anthropometric and blood pressure assessments
Body weight was measured with light clothing and without shoes on a balance scale. Height was measured without shoes and caps using a meter that was supported on the balance scale and BMI was calculated as weight in kilograms divided by height squared in meters. Waist circumference was measured in centimeters using a flexible measuring tape in a horizontal plane midway between the iliac crest and the costal margin at the end of expiration. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured as millimeters mercury by a mercury sphygmomanometer while the patient was relaxed, comfortably seated, and with well supported arm.
Biochemical assays
All biochemical parameters were determined after 12 hours overnight fasting. Fasting blood samples were collected at the morning between 8.00 am and 10.00 am. Seven mL of blood were withdrawn by a sterile syringe from the antecubital vein. Complete aseptic technique was used with safe disposal of materials used.
Glycated hemoglobin (HbA1c) was measured quantitatively by spectrophotometric method using commercial kits (Stanbio Laboratory Company, Texas, USA) [13]. Regarding serum lipid profile, TC was determined by enzymatic colorimetric method [14], TGs was determined by enzymatic colorimetric method [15], and HDL-C was determined by precipitation method [16] using commercial kits (Spinreact Company, Girona, Spain). While, LDL-C was calculated using the Friedewald’s formula: LDL-C (mg/dL) = TC (mg/dl) – [TGs (mg/dl)/5] - HDL-C (mg/ dl) in case of TGs concentration is ˂400 mg/dl [17]. Serum level of hs-CRP was determined by a solid phase enzyme-linked immunosorbent assay (ELISA) using commercial kits (Diagnostic Automation Inc. company, California,USA) [18]. The TAC was determined by enzymatic colorimetric method using commercial kits (Biodiagnostic Company, Giza, Egypt) [19]. The level of PAI-1 was determined by ELISA assay using commercial kits (Affymetrix eBioscience company, Vienna, Austria) [20].
All participants were assessed for the anthropometric data, SBP, DBP, and biochemical parameters twice: at baseline (after titration period) and at the endpoint of week 12.
Statistical analysis
Results were analyzed using the Statistical Package for Social Science (SPSS) version 22 (August 2013), IBM corporation software group, Chicago, USA. Data were expressed as mean ± standard deviation (SD) for continuous variables and percentage for categorical variables. Comparisons within and between groups were performed using Student’s t-test. Chi-square test was used for comparisons between categorical variables. Binary logistic regression analysis was performed to illustrate the effect of independent predictors (vildagliptin/metformin versus metformin and change in HbA1c levels) on the outcome of the significantly changed biological markers. In all cases, a two-tailed p-value less than 0.05 were considered statistically significant.
Baseline characteristics of patients
No statistically significant differences were observed between both studied groups in demographic, anthropometric, and all other studied parameters at baseline (Table 1).
| Parameters | Metformin group | Vildagliptin/metformin group | p-value |
|---|---|---|---|
| (n=20) | (n=20) | ||
| Number (male/female) | Jun-14 | Jul-13 | 0.74 |
| Smoking % | 10% | 15% | 0.63 |
| Age (years) | 54.45 ± 5.05 | 53.2 ± 6.82 | 0.51 |
| Height (m) | 1.6 ± 0.05 | 1.62 ± 0.06 | 0.26 |
| Body weight (kg) | 91.21 ± 12.56 | 91.15 ± 11.74 | 0.99 |
| BMI (kg/m2) | 35.46 ± 4.82 | 34.79 ± 5.72 | 0.69 |
| Waist circumference (cm) | 123.65 ± 10.67 | 121.6 ± 9.67 | 0.53 |
| Baseline medications (%): | |||
| Statins | 45% | 50% | 0.75 |
| Antihypertensive | |||
| ACE inhibitors | 20% | 10% | 0.38 |
| ARBs | 10% | 10% | 1 |
| CCBs | 0 | 5% | 0.31 |
| β-blockers | 5% | 0% | 0.31 |
| Antiplatelet | 10% | 0% | 0.15 |
| HbA1c (%) | 9.53 ± 1.3 | 9.99 ± 1.35 | 0.28 |
| hs-CRP (mg/L) | 3.94 ± 2.22 | 4.71 ± 2.5 | 0.31 |
| TC (mg/dl) | 186.25 ±27.34 | 191 ± 33.34 | 0.63 |
| TGs (mg/dl) | 137.6 ± 21.51 | 145.55 ± 23.98 | 0.28 |
| HDL-C (mg/dl) | 48.95 ± 7.19 | 45.1 ± 10.6 | 0.19 |
| LDL-C (mg/dl) | 109.78 ± 30.55 | 116.79 ± 39.37 | 0.53 |
| PAI-1 (ng/ml) | 85.35 ± 14.78 | 85.95 ± 18.03 | 0.91 |
| TAC (mM/L) | 0.85 ± 0.35 | 0.98 ± 0.36 | 0.23 |
| SBP (mmHg) | 126.75 ± 14.89 | 125.5 ± 17.39 | 0.81 |
| DBP (mmHg) | 83 ± 7.33 | 85.5 ± 10.5 | 0.39 |
Data are presented as means ± standard deviation for continuous variables and percentage for categorical variables. BMI: body mass index; ACE: angiotensin converting enzyme; ARBs: angiotensin receptor blockers; CCBs: calcium channel blockers. HbA1c: glycated hemoglobin A1c; hs-CRP: high-sensitivity C-reactive protein; TC: total cholesterol; TGs: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; PAI-1: plasminogen activator inhibitor-1; TAC: total antioxidant capacity; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Table 1: Baseline characteristics of the patients.
Effects on anthropometric and BP measurements
After 12 weeks of metformin therapy, there were no significant changes observed in anthropometric measurements, SBP, and DBP compared to their levels before treatment. After combined vildagliptin/metformin therapy, there were significant reductions in waist circumference, SBP, and DBP levels, while there were no significant changes in body weight and BMI. When comparing the two groups after treatment, there were no significant differences observed in anthropometric measurements, there were significant differences between both treatment groups in SBP (Δ change: -12.5 ± 13.03 mmHg after combined therapy versus -3.75 ± 11.57 mmHg after metformin monotherapy, p= 0.012) and DPB (Δ change: -10.25 ± 9.39 after combined therapy versus -2.5 ± 9.39 mmHg after monotherapy, p= 0.009) (Table 2).
| Parameters | Metformin group | P1 | Vildagliptin/metformin group | P1 | P2 | ||
|---|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | Baseline | After 12 weeks | ||||
| Body weight (kg) | 91.21 ± 12.56 | 90.7 ± 11.87 | 0.46 | 91.15 ± 11.74 | 90.29 ± 10.92 | 0.26 | 0.91 |
| BMI (kg/m2) | 35.46 ± 4.82 | 35.27 ± 4.59 | 0.45 | 34.79 ± 5.72 | 34.48 ± 5.51 | 0.25 | 0.63 |
| Waist circumference (cm) | 123.65 ± 10.67 | 123.05 ± 10.35 | 0.28 | 121.6 ± 9.67 | 120.05 ± 9.83 | 0.024 | 0.35 |
| SBP (mmHg) | 126.75 ± 14.89 | 123 ± 12.18 | 0.16 | 125.5 ± 17.39 | 113 ± 11.74 | 0.000 | 0.012 |
| DBP (mmHg) | 83 ± 7.33 | 80.5 ± 5.83 | 0.25 | 85.5 ± 10.5 | 75.25 ± 6.17 | 0.000 | 0.009 |
Data are presented as means ± SD. BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; P1: p-value within group; P2: p-value between groups after treatment.
Table 2: Effect of treatment on anthropometric and BP measurements.
Effects on HbA1c and lipid profile
After metformin therapy, there was a significant reduction in HbA1c with no significant changes in lipid parameters. While after combined vildagliptin/metformin therapy, there were significant improvements in HbA1c, TGs, HDL-C, there were no significant changes in TC and LDL-C. When comparing the two groups after treatment, the reduction of HbA1c in combined group was significantly greater than that in metformin group (Δ change after 12 weeks: -2.68 ± 2.24% versus -1.37 ± 1.8%, P = 0.043, respectively). In addition, HbA1c was ˂ 7% in 45% of cases in combined group compared to 30% of cases in metformin group with no statistically significant differences between both groups. There were no significant differences between both groups in lipid parameters except for TGs (Δ change: -9.7 ± 18.48 mg/dl after combined therapy versus 10.35 ± 27.36 mg/dl after metformin monotherapy with p= 0.037) (Table 3).
| Parameter | Metformin group | P1 | Vildagliptin/metformin group | P1 | P2 | ||
|---|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | Baseline | After 12 weeks | ||||
| HbA1c % | 9.53 ± 1.3 | 8.16 ± 1.27 | 0.003 | 9.99 ± 1.35 | 7.31 ± 1.28 | 0.000 | 0.043 |
| TC (mg/dl) | 186.25 ± 27.34 | 181.15 ± 20.01 | 0.43 | 191 ± 33.34 | 176.1 ± 12.59 | 0.054 | 0.35 |
| TGs (mg/dl) | 137.6 ± 21.51 | 147.95 ± 17.08 | 0.11 | 145.55 ± 23.98 | 135.85 ± 18.33 | 0.03 | 0.037 |
| HDL-C (mg/dl) | 48.95 ± 7.19 | 49.5 ± 5.16 | 0.75 | 45.1 ± 10.6 | 49.9 ± 5.24 | 0.049 | 0.81 |
| LDL-C (mg/dl) | 109.78 ± 30.55 | 102.06 ± 22.12 | 0.28 | 116.79 ± 39.37 | 99.03 ± 13.88 | 0.06 | 0.61 |
Data are presented as means ± SD. HbA1c: glycated hemoglobin; TC: total cholesterol; TGs: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; P1: p-value within group; P2: p-value between groups after treatment.
Table 3: Effect of treatment on HbA1c and lipid profile.
Effects on, TAC, hs-CRP, and PAI-1
After 12 weeks of treatment, metformin therapy showed significant increase in TAC (Δ change: 0.18 ± 0.22 mM/L, p= 0.002), significant decrease in hs-CRP (Δ change: -1.11 ± 1.78 mg/L, p= 0.012), and small non- significant increase in PAI-1 (Δ change: 3.9 ± 19.39 ng/ ml, p= 0.38). The combined therapy significantly increased TAC (Δ change: 0.21 ± 0.23 mM/L, p= 0.001), significantly decreased hs-CRP (Δ change: - 1.9 ± 3.64 mg/L, p= 0.031), and reduced PAI- 1 non-significantly (Δ change: -7.93 ± 17.11 ng/ml, p= 0.052). By comparing the two groups, there were no significant differences observed in TAC and hs-CRP, while there were significant changes in PAI-1 (p= 0.048) (Table 4).
| Parameter | Metformin group | P1 | Vildagliptin/metformin group | P1 | P2 | ||
|---|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | Baseline | After 12 weeks | ||||
| TAC (mM/L) | 0.85 ± 0.35 | 1.03 ± 0.36 | 0.002 | 0.98 ± 0.36 | 1.19 ± 0.46 | 0.001 | 0.23 |
| hs-CRP (mg/L) PAI-1 (ng/ml) |
3.94 ± 2.22 85.35 ± 14.78 |
2.83 ± 1.48 89.25 ± 15.08 |
0.012 0.38 |
4.71± 2.5 85.95 ± 18.03 |
2.81 ± 2.65 78.03 ± 19.44 |
0.031 | 0.97 |
| 0.052 | 0.048 | ||||||
Data are presented as means ± SD. TAC: total antioxidant capacity; hs-CRP: high-sensitivity C-reactive protein; PAI-1: plasminogen activator inhibitor-1; P1: p-value within group; P2: p-value between groups after treatment.
Table 4: Effect of treatment on TAC, hs-CRP, and PAI-1.
Logistic regression analysis
Results from binary logistic regression analysis revealed that adding vildagliptin to metformin was independently associated with the significant reduction in TGs [exponentiation of the B coefficient: Exp (B)= 5.35, p= 0.023] rather than that for the change in HbA1c levels [Exp (B)= 5.43, p= 0.16]. On the other hand, neither treatment modality nor changes in HbA1c levels were independently associated with the reduction in PAI-1 levels between groups at the end of the study period (p≥ 0.05).
Treatment adverse effects
During treatment period, both treatment lines were generally well tolerated with no serious side effects. A number of mild side effects were recorded as illustrated in Figure 1 with no statistically significant differences between both studied groups.
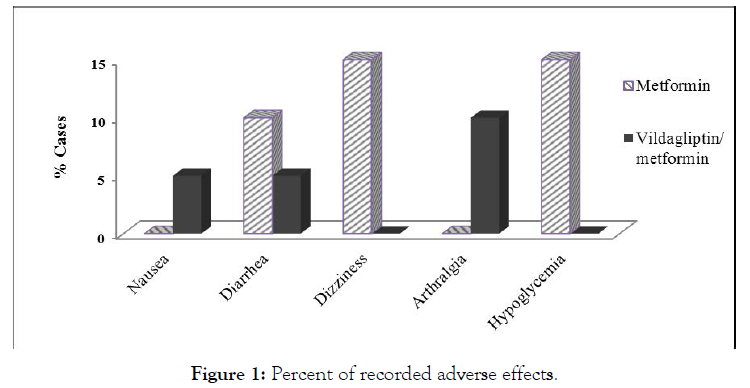
Figure 1: Percent of recorded adverse effects.
The main findings of this study were that adding vildagliptin to metformin therapy reduced SBP, DBP, HbA1c, TGs, and PA1- 1 levels as compared to metformin monotherapy with no risk of hypoglycemic events in newly diagnosed patients with T2DM. Vildagliptin showed no effects on TAC, hs-CR, and other lipid parameters.
Previous studies demonstrated that combination therapy of metformin plus vildagliptin significantly reduced HbA1c levels compared to monotherapy of each one [21, 22]. Results from the present study supported these findings. Underlying mechanism may be mediated by the glycemic control augmentation of vildagliptin and metformin with the enhanced stimulation of postprandial insulin secretion. In addition, metformin beyond its known action of reducing hepatic glucose output and improving insulin resistance has been shown to stimulate the GLP-1 biosynthesis in preclinical studies [23]. Furthermore, the levels of intact GLP-1 can be preserved by the inhibitory action of vildagliptin on DPP-4 enzyme.
While, clinical data regarding the effect of vildagliptin on SBP and DBP levels remain uncertain and driven mainly form preclinical and mechanistic studies. According to the present study, vildagliptin therapy for 12 weeks was associated with significant reductions in SBP and DBP levels. This effect may contribute to a great advantage for patients with T2DM. The vasodilatory effect of vildagliptin may be mediated by the effect of GLP-1 on the nitric oxide production with endothelium dependent relaxations as shown by Liu et al [24]. Moreover, vildagliptin has been shown to exert diuretic effects according to recent preclinical data [25]. In addition, Shah et al. demonstrated the direct effect of alogliptin therapy on the nitric oxide system, independent of GLP-1, with subsequent vascular tone relaxation in animal models [26].
Atherogenic dyslipidemia is a metabolic disorder of hyperglycemia which increases the CV risk in diabetic population [27]. In addition, hypertriglyceridemia was shown to elevate the risk of myocardial infarction, ischemic heart disease, and death as reported by the prospective cohort study in Denmark [28]. The clinical implications of the effect of vildagliptin therapy on lipoprotein metabolism are still controversial. According to the present study, positive effects of vildagliptin on the lipid profile levels were shown. After 12 weeks, vildagliptin was shown to exert a significant reduction in fasting levels of TGs beyond its glycemic control. In addition, the combined therapy was associated with a trend for better improvement of the other studied lipid parameters as compared to metformin monotherapy but without reaching significance.
These findings are in consistence with Matikainen et al. who demonstrated that vildagliptin therapy for four weeks reduced postprandial plasma TGs and apolipoprotein B48 in drug naïve patients with T2DM [29]. Hsieh et al. also provided the evidence of DPP-4 inhibitors effect on controlling postprandial lipoprotein biosynthesis and secretion through GLP‑1 receptor signaling in animal model [30]. On the other hand, slight non-significant increase in fasting TGs was shown after metformin therapy. Further explanations of the underlying cause are needed.
According to the atherothrombotic CV risk factor, PAI-1, this study demonstrated that combined therapy was associated with significant reduction in PAI-1 levels compared with metformin therapy which the later was associated with slight increase in PAI-1 levels. These findings are in consistence with the work by Strózik et al. work who found that PAI-1 levels increased in patients treated with metformin while these levels decreased after adding vildagliptin to the metformin therapy [31]. Underlying mechanism of the favorable effect of vildagliptin on PAI-1 is still unexplained and unclear. However it may be supported by data from previous preclinical studies which demonstrated the effect of vildagliptin on the reduction of PAI-1 gene expression in rat thoracic aorta and retina [32, 33].
Hypoglycemia is associated with an increased risk of CVD [34]. Unfortunately, most commonly used antihyperglycemic agents have been reported to be associated with increased risk of hypoglycemia [35, 36]. In this work, both treatment approaches were shown to be generally well tolerated. No case of hypoglycemia was reported in vildagliptin/metformin group compared to three cases (15%) in metformin group during the treatment period. Previous literatures supported that metformin monotherapy is associated with hypoglycemic risk of zero to 21% [35].
On the other hand, incretin hormones regulate glucose hemostasis in glucose dependent manner lowering the probability of hypoglycemia [37]. In addition, according to previous clinical data, vildagliptin was shown to inhibit glucagon levels during the hyperglycemic state while it sustained glucagon counter-regulation at lower levels of glycemia [38].
Moreover, Results from this work revealed that vildagliptin is weight neutral and hasn’t been associated with increasing BMI. These findings are in consistence with previous studies [39,40]. Regarding the central obesity, Valerio et al. demonstrated a positive correlation between DPP-4 enzyme levels and central fat distribution in patients with familial partial lipodystrophy type 2 [41]. Thus, inhibition of DPP-4 enzyme may be associated with central fat redistribution and waist circumference reduction. Our work revealed that the combined therapy was associated with a significant reduction in waist circumference while as compared to metformin therapy this reduction didn’t reach the significance. Similarly, vildagliptin improved hs-CRP and TAC levels without significance. Small sample size and short duration of follow up which are the major limitations of this study may contribute to these results.
In conclusion, vildagliptin was shown to be weight neutral, with no risk of hypoglycemia, and with a good impact on some atherogenic risk parameters including SBP, DBP, TGs, and PAI-1. Vildagliptin therapy did not affect hs-CRP and TAC levels significantly. These findings may reflect possible beneficial effects of adding vildagliptin to metformin therapy for more glycemic control and with possible additional CV risk benefits in patients with T2DM and CV risk. Further large randomized controlled trials with larger sample size and longer duration of therapy are needed to confirm our results and explore more beneficial aspects of vildagliptin on other atherosclerotic CV markers.
The authors thank Professor Amal El-Bendary, Clinical Pathology Department, Faculty of Medicine, Tanta University, Egypt, for her co-operation in laboratory analysis. Great thanks for Dr. Ibrahim Kabbash, Professor of Public Health and Biostatistics, Faculty of Medicine, Tanta University, Egypt, for his assistance in the regression analysis.
Conflicts of interest: there were no conflicts of interest that any of the authors may have.
Citation: Bassant MM, El-Haggar SM, Abdelraouf YM, Elazab GA (2021) The Effect of Atherothrombotic Markers in Newly Diagnosed Patients with Type 2 Diabetes Mellitus. J Diabetes Metab. 12:907. doi: 10.35248/2252-5211.21.12.907
Received: 09-Nov-2021 Published: 30-Nov-2021
Copyright: © 2021 Bassant MM, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.