Research Article - (2019) Volume 10, Issue 9
Diabetes mellitus, hyperglycemia and polyuria, results from the body either producing insufficient insulin or using the produced insulin inefficiently to metabolize glucose. Type I diabetes mellitus (T1DM) is recognized as reduction of beta-cell mass mostly due to autoimmune destruction. However, insulin resistance and beta-cell dysfunction or loss are potentially the main causes of Type II diabetes mellitus (T2DM). Due to long-term hyperglycemia and microvascular impairment, complications are common, including heart, kidney, liver and other organs. Therefore, diabetic patients have the overall high risk of dying prematurely by heart attack, stroke, liver dysfunction and kidney failure. Diabetes has become one of the major global threats to human health. Development of safe and potent therapeutics is thus urgently needed. Animal models remain irreplaceable for discovering, validating and optimizing novel therapeutics for their safe use in clinics. Selection of appropriate animal models is critical for obtaining crucial data to translate preclinical research to clinical trial. It has been recognized that the pancreas structure and pathophysiology in rodents greatly differ from those in humans, but in nonhuman primates (NHPs) are more similar to humans. Many researchers have used spontaneously developed or drug-induced diabetic NHPs as the models to investigate diabetes progression and novel therapies. This article summarized some characteristics of the disease progression in a large pool of diabetic NHPs.
Diabetes; Nonhuman primate; Beta cell; Diabetes progression
Obesity has become a worldwide epidemic issue and can lead to diabetes because of insulin resistance. Several hypotheses for insulin resistance have been postulated and documented. Among those are inflammation, mitochondrial dysfunction, hyperinsulinemia and lipotoxicity [1,2]. Others, such as genetic background, aging, fatty liver, endoplasmic reticulum stress, hypoxia and lipodystrophy are the areas under active research for understanding mechanisms of insulin resistance [1]. Pancreatic beta-cell dysfunction and loss are the key pathophysiological factors in development of diabetes mellitus (type 1 and type 2). Due to relatively insulin deficiency and unable to properly metabolize glucose, diabetic patients manifest hyperglycemia and polyuria. The world prevalence of diabetes among adults (aged 20–79 years) are predicted to be 439 million by 2030 [3], which becomes a major threat to human health. If without proper treatment, diabetic patients can eventually develop diabetic complications of the kidneys, liver, heart and other organs, which may significantly impact on their quality of life.
Potential new therapies and technologies will help to improve the quality of life beyond current standard of care and perhaps even to cure the disease in future [4,5]. Various animal models have been used in preclinical research for understanding the disease and discovering new novel therapies [6-9]. NHPs can naturally develop T2DM [10-15]. Therefore, diabetes in NHPs represents a crucial pre-clinical model with important similarity to human endocrine physiology that facilitates translation of experimental findings to clinic. Diabetes accelerates the atherosclerotic process and its morbid consequences. Nutraceuticals and functional food ingredients may potentially reduce the overall cardiovascular risk from dyslipidemia [16]. Compared with humans, NHPs are more accessible and feasible for biopsy and tissue/organ collection for histopathology. NHP models are also used for investigation of diabetic complications and discovery of new mechanism and therapeutic strategy or target [17,18].
Though diabetes can be treated with oral antidiabetic drugs or subcutaneous insulin injection, these treatments do not provide the same degree of glycemic control as functional pancreatic β-cells and do not prevent the debilitating consequences of the disease. Treatments that replenish β-cell mass in diabetic patients could allow for the long-term restoration of normal glycemic control and thus represent a potentially curative therapy. It has been known that the pancreas pathophysiology in rodents differed from that in humans greatly, but in NHPs was more close to in humans [19-21]. Streptozocin (STZ)-induced diabetes in NHPs is also used to investigate diabetes progression and therapy [22-26], because naturally developed diabetes may take years to show the phenotype and raise the difficulty to monitor the disease progress. STZ-induced diabetes can be predicted in a short period of time. Due to limited availability of spontaneously diabetic NHPs and other specific reasons, drug-induced diabetes in NHPs is also highly valuable for scientific research on diabetic etiology and its complications. STZinduced diabetes can give us the relative convenience of monitoring the dynamic changes from non-diabetes to diabetes in a designated duration. In addition, islet transplantation is an attractive treatment for diabetes, especially for Type I. Therefore, STZ-induced diabetes in NHPs is one of suitable models for evaluating the effectiveness of islet transplantation [15,27].
This article summarizes some characteristics of naturally occurring or drug-induced diabetes in a large pool of NHPs. Normal and obese/diabetic monkeys housed in our animal center are collected periodically from the monkey farms in China and then raised in our own facility [28-30]. Monkeys raised in indoor cages with food ad libitum increase the chance to become obesity which facilitates development of obesity-associated diseases in an age-dependent manner [31-34]. Like humans, these monkeys can develop to Type 2 diabetes mellitus and other complications, such as nephropathy, orthmopathy, neuropathy, liver and cardiovascular complications [12,31,35].
Experiments were performed in normoglycemic and hyperglycemic cynomolgus monkeys of either sex (Table 1). The diabetic monkeys were selected from spontaneously developed diabetic ones who met the diabetes criteria and were housed in our animal facility [10]. These monkeys were individually housed and maintained in our animal facility in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The room temperature was maintained at ∼21°C with a 12 hrs light/dark cycle with lights on from 7 AM to 7 PM. All the animals were free access ad libitum to water and a complete, nutritionally balanced normal diet (Beijing Keao Xieli Feed Co., LTD, Beijing, China) enriched with seasonal fruit and vegetables. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Crown Bioscience, Inc.
Table 1: General characteristics of the normoglycemia and hyperglycemia NHPs.
| Parameter | Normoglycemia | Hyperglycemia | p value |
|---|---|---|---|
| (M ± SE (n, F/M)) | (M ± SE (n, F/M)) | ||
| Age (year) | 13.5 ± 0.2 (342, 9/333) | 15.8 ± 0.2 (801, 179/622) | <0.0001**** |
| Body weight (kg) | 9.4 ± 0.1 (342, 9/333) | 8.9 ± 0.1 (801, 179/622) | 0.0009 *** |
| Glucose (mg/dL) | 69.8 ± 0.5 (342, 9/333) | 190.2 ± 3.6 (704, 141/563) | <0.0001**** |
| Insulin (mIU/L) | 104.8 ± 9.7 (236, 7/229) | 101.0 ± 9.1 (561, 112/449) | 0.7772 |
| C-peptide (nmol/L) | 1.5 ± 0.1 (82, 1/81) | 1.5± 0.1 (190, 37/153) | 0.9649 |
| HbA1c (%) | 4.8 ± 0.1 (173, 5/168) | 8.7 ± 0.1 (525, 95/430) | <0.0001**** |
| TG (mg/dL) | 100.3 ± 9.8 (267, 7/260) | 243.8 ± 11.4 (610, 106/504) | <0.0001**** |
| TC (mg/dL) | 134.2 ± 9.6 (265, 7/258) | 155.6 ± 5.2 (612, 106/506) | 0.0052** |
| HDL (mg/dL) | 62.5 ± 2.1 (252, 7/250) | 45.4 ± 1.1 (516, 79/437) | <0.0001**** |
| LDL (mg/dL) | 67.5 ± 5.3 (254, 7/247) | 77.8 ± 4.4 (520, 79/441) | 0.1333 |
| Leptin (ng/mL) | 24.8 ± 0.7 (11, 1/10) | 23.4 ± 0.9 (12, 6/6) | 0.237 |
| proBNP (ng/mL) | 11.8 ± 1.4 (8, 0/8) | 9.5 ± 1.1 (24, 12/12) | 0.209 |
| ivGTT AUC (mg/dL*min) | 4523 ± 563 (8, 0/8) | 7274 ± 415 (24, 12/12) | 0.0012** |
| oGTT AUC (mg/dL*min) | 20626 ± 3349 (8, 0/8) | 44086 ± 2552 (90, 10/80) | <0.0001**** |
Note: M ± SE, mean ± the standard error of the mean; n: the number of animals; F/M: Female/Male; *: p<0.05; **: p<0.01: ***: p<0.001; ****: p<0.0001; versus Normoglycemia.
To obtain the parameters showed in Tables and Figures in this manuscript, each overnight-fasted (approximately 16 hrs) monkey sit in a monkey chair. The body weight was measured and recorded. Blood (2 mL/each) was collected from a cephalic or saphenous vein into BD Vacutainer® SST II Plus plastic serum tubes. The animal was then returned to its cage after blood collection. The sample tube was left at room temperature (approximately 21°C) for 30 min. Serum was separated by centrifugation at 3000 rpm for 15 min at 4°C. Another blood sample was kept at 4°C for glycated hemoglobin (Hb1Ac) assay. All the samples were analyzed within 3 to 4 hrs after blood collection. Blood HbA1c (by ionexchange HPLC), serum lipids, glucose (by Siemens Advia-2400) and insulin (by Siemens Advia Centaur XP) were analyzed at the clinical laboratory (The First People’s Hospital of Taicang, Taicang, Jiangsu Province, China). Serum leptin and pro-BNP (pro b-type natriuretic peptide) were measured with immunoassay (ELISA Kits, MyBioSource, San Diego, California, USA) specifically targeting the monkey.
Intravenous glucose tolerance test (ivGTT) was conducted in our study to evaluate the metabolic stages of the monkeys [36,37]. The monkeys were objectively divided into 2 distinct groups based on their serum glucose levels (Table 1), normoglycemia (<85 mg/dL, n=8) and hyperglycemia (≥ 85 mg/dL, n=24). The animals were fasted for overnight 16 hrs and anesthetized with an initial dose of ketamine at 10 mg/kg intramuscularly (i.m.) and then with 5 mg/kg supplement if needed. The cephalic and/or saphenous veins were cannulated separately for glucose infusion and blood collection. Glucose (0.25 g/kg=0.5 mL/kg of 50% dextrose) was intravenously infused within a 30 second period and the system was flushed with 5 mL saline to remove residual glucose. Blood was collected immediately before and then at 3, 5, 7, 10, 15, 20, 30 min after glucose infusion. Collected blood samples were kept at room temperature (~21°C) for 30 min and then centrifuged at 3,500 rpm for 10 min to obtain the serum. Serum glucose and insulin were measured in the clinical laboratory (Taicang 1st People’s Hospital, Jiangsu, China).
For oral glucose tolerance test (oGTT), each monkey was overnight fasted for approximately 16 hrs. After being placed in a monkey chair, a nasal/oral gavage tube was placed into the stomach. Glucose 1.75 g/kg in 35% glucose solution was administered via the gavage tube at 5 mL/kg in each conscious animal followed by flashing with 5 mL 0.9% saline to wash the residual glucose solution into the stomach. Blood samples were collected and processed. Serum glucose and insulin levels were measured before and 15, 30, 60, 90, 120, 180 min after glucose administration. The animal was placed back to its cage from the monkey chair after completion of oGTT. Serum glucose and insulin were measured in the clinical laboratory (Taicang 1st People’s Hospital, Jiangsu, China).
General characteristics of spontaneous diabetes in NHPs
Some metabolic characteristics of the male and female monkeys being housed in our animal facility are shown in Figure 1. Clearly, compared to the male monkeys, the female ones were significantly older (Figure 1A, p<0.001) and showed significantly higher glucose and HbA1c levels (Figures 1C and 1D, p<0.001). The serum proBNP and leptin levels were very similar between female and male monkeys (p>0.05; Figures 1G and 1H). In contrast, the body weight (p<0.001), insulin (p<0.001) and C-peptide (p<0.01) levels were significantly lower in the female monkeys than in the male ones (Figures 1B, 1E and 1F). The data indicate that compared with the male monkeys, our housed female ones were older and showed more late stage diabetes (Figure 1). Most of the monkeys enrolled in this study are currently alive and have been housed in our animal facility for diabetes and dyslipidemia research for many years.
Figure 1: The differences of age (F/M (Female/Male)=172/971), body weight (F/M=180/978), serum glucose (F/M=149/899), blood HbA1c (F/ M=97/601), serum insulin (F/M=119/678), C-peptide (F/M=53/219), porBNP (F/M=11/21) and leptin (F/M=8/34) between female and male monkeys. **, p < 0.01; ***, p < 0.001; vs. Female.
Multiple reports clearly show that normal fasting serum glucose concentrations in monkeys are about 30 mg/dL lower than those in normal humans [10,12,17]. The monkeys housed in our facility with their fasting serum glucose <85 mg/dL (69.8 ± 0.5 mg/dL, mean ± SE, n=342, Table 1) were listed in the normoglycemic group. Those animals showed their fasting serum glucose levels equal or higher than 85 mg/dL were placed in the hyperglycemic group (190.2 ± 3.6 mg/dL, n=704, p<0.05, vs. Normoglycemia, Table 1). In the meantime, compared with normoglycemic animals, the hyperglycemic monkeys were significantly older with higher blood HbA1c, TG (triglycerides), TC (total cholesterol), ivGTT AUC (area under the curve), and oGTT AUC (p<0.05 to 0.0001, Table 1). In contrast, body weight and serum HDL (high density lipoprotein) level were significantly lower in the hyperglycemic group than in the normoglycemic group (p<0.001 to 0.0001, Table 1). Interestingly, serum insulin, C-peptide, LDL (low density lipoprotein), leptin and pro-BNP levels were not significantly different between normoglycemic and hyperglycemic groups (p>0.05, Table 1). Our data demonstrate that diabetic monkeys were much older than normoglycemic ones in our animal facility. Compared with normoglycemic monkeys, diabetics ones showed significantly lower body weight with higher glucose, HbA1c, TG, TC, ivGTT AUC and oGTT AUC.
Correlations among age, body weight and serum/urine parameters
The ages of the experimental animals correlated significantly well with their serum glucose (Figure 2A, p<0.001) and blood HbA1c (Figure 2B, p<0.0001) levels. Their ages did not correlate well with their serum insulin levels (Figure 2D, p>0.05), but negatively correlated well with serum C-peptide levels (Figure 2C, p<0.05). Interestingly, the body weights, not like their ages (Figures 2C and 2D), of the animals showed significant correlation with serum C-peptide (Figure 2G, p<0.001) and insulin (Figure 2H, p<0.0001) levels. In addition, their body weights negatively correlated with serum glucose (Figure 2E, p<0.001) and blood HbA1c (Figure 2F, p<0.001) levels. Our results indicate that when the animals got older, hyperglycemia and high HbA1C became more severe, but serum insulin and C-peptide levels were decreased because of dysfunction and/or loss of pancreatic beta-cell masses due to impairment of long time hyperglycemia. However, as obesity could cause insulin resistance with compensational more insulin secretion and as diabetic NHPs at their late stages might lose their body weights and pancreatic beta-cell masses, the insulin and C-peptide levels thus correlated positively with the body weights in the experimental NHPs (Figure 2). In addition, due to the similar reasons, the glucose and HbA1c levels positively correlated with the ages, but negatively correlated with body weights (Figure 2).
Figure 2: Correlations between age and serum glucose (A, n=865, p < 0.01), HbA1c (B, n=526, p < 0.0001), C-peptide (C, n=188, p < 0.05) and insulin (D, n=613, p>0.05). Correlations between body weight and serum glucose (E, n=878, p < 0.01), HbA1c (F, n=572, p < 0.001), insulin (F, n=646, p < 0.0001), C-peptide (G, n=230, p < 0.001) and insulin (H, n=646, p < 0.0001).
Blood glucose in the bloodstream is transported to all cells in the body as one of the energy sources. A healthy person needs to keep blood levels within a safe range to prevent the risk of diabetes and heart disease. Hyperglycemia is a major concern for those who suffer from type 1 or type 2 diabetes. In order to learn about the effects of blood glucose levels on other blood biomedical parameters and renal functions, the correlations between serum glucose levels and other blood and urine parameters were analyzed. The results of the correlations and statistical significances are shown in Table 2. Clearly, the serum glucose levels did not correlate well with the serum insulin levels, because some diabetic animals at their late stages had much high blood glucose accompanied with significantly low insulin. The potential explanation is long-term hyperglycemia in diabetic NHPs, especial at late stage, led to pancreatic beta-cell dysfunction and loss, which decreases the capability of the pancreas to synthesize and secret insulin.
Correlation |
R | p |
|---|---|---|
| Serum glucose levels/other parameters | ||
| Glucose (mg/dL)/Insulin (mIU/L) (n=787) | -0.05 | >0.05 |
| Glucose (mg/dL)/C-peptide (nmol/L) (n=271) | -0.13 | <0.05 |
| Glucose (mg/dL)/HbA1c (%) (n=690) | 0.72 | <0.0001 |
| Glucose (mg/dL)/TG (mg/dL) (n=845) | 0.43 | <0.0001 |
| Glucose (mg/dL)/TC (mg/dL) (n=845) | 0.146 | <0.0001 |
| Glucose (mg/dL)/LDL (mg/dL) (n=751) | 0.09 | <0.05 |
| Glucose (mg/dL)/HDL (mg/dL) (n=745) | -0.27 | <0.0001 |
| Glucose (mg/dL)/ALT (U/L) (n=547) | 0.43 | <0.0001 |
| Glucose (mg/dL)/AST (U/L) (n=488) | 0.18 | <0.0001 |
| Glucose (mg/dL)/FI (g/24) (n=321) | 0.2 | <0.0001 |
| Serum glucose levels/Urine parameters | ||
| Glucose (mg/dL)/Volume (mL/24 hrs) (n=94) | 0.25 | <0.05 |
| Glucose (mg/dL)/Glucose (mg/24 hrs) (n=79) | 0.41 | <0.001 |
| Glucose (mg/dL)/Albumin (mg/24 hrs) (n=97) | 0.14 | >0.05 |
| Glucose (mg/dL)/Total proteins (mg/24 hrs) (n=98) | 0.13 | >0.05 |
| Glucose (mg/dL)/Creatinine (mmol/24 hrs) (n=91) | 0.09 | >0.05 |
| Glucose (mg/dL)/Albumin creatinine ratio (n=100) | 0.18 | >0.05 |
Note: Data points of two corresponding parameters were fitted by linear regression with the equation Y=A+B*X (Microcal Origin 6.0, OriginLab Corporation, Northampton, MA , USA). The correlation (R value) and statistical significance (pvalue) was indicated after linear fitting.
Table 2: Correlations between serum glucose levels and other biochemical parameters.
The correlations between serum glucose levels and urine several parameters were also analyzed (Table 2). Interestingly, the serum glucose levels in the NHPs correlated well with the urine volumes (p<0.05) and glucose amounts (p<0.001) collected in 24 hrs. However, the correlations between serum glucose levels and the amounts of urine albumin, total proteins, creatinine or albumin/ creatinine ratio were not statistically significant (p>0.05, Table 2). These results indicate that higher blood glucose levels could lead to increase in urine volume and glucose execration, but might not represent seriousness levels of diabetic nephropathy.
The kidney functions were further compared between normoglycemia (<85 mg/dL) and hyperglycemia (≥ 85 mg/ dL) NHPs (Table 3). Compared with the normal monkeys, the hyperglycemic animals showed significant less body weight, older, high serum glucose, high HbA1c and ALT. Urine assays showed significant increase in 24 hrs urine volume, albumin, total proteins, creatinine and albumin/creatinine ration (p<0.001, Table 3). These results demonstrate that diabetes occurs more in aged NHPs and may susceptibly affect liver function. In the meantime, liver and renal functions were impaired in diabetic animals.
Parameter |
Normoglycemia | Hyperglycemia |
|---|---|---|
| (n=21, F/M=0/21) | (n=79, F/M=24/55) | |
| Age (year) | 13.3 ± 0.5 | 17.3 ± 0.5**** |
| Body weight (kg) | 10.8 ± 0.4 | 8.1 ± 0.3**** |
| Serum glucose (mg/dL) | 66.9 ± 1.8 | 240.1 ± 13.4**** |
| Blood HbA1c (%) | 5.2 ± 0.7 | 9.7± 0.4** |
| Serum ALT (U/L) | 41.3 ± 7.7 | 139.5± 21.6*** |
| Serum AST (U/L) | 34.1 ± 2.5 | 42.1 ± 3.9 |
| Urine assay | ||
| Volume (mL/24 hrs) | 340 ± 41 | 583 ± 43*** |
| Albumin (mg/24 hrs) | 4.9 ± 1.1 | 69.8 ± 13.9**** |
| Total proteins (mg/24 hrs) | 21.1 ± 3.0 | 140.8 ± 26.7**** |
| Creatinine (mmol/24h) | 2.0 ± 0.2 | 916.2 ± 289.1**** |
| Albumin/Creatinine ratio | 2.7 ± 0.6 | 56.2 ± 9.7**** |
Note: Values are presented as Mean ± SE. n: number of animals; F/M: Female/Male; *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001; versus Normoglycemia.
Table 3: Urine parameters in normoglycemia and hyperglycemia NHPs.
General progression and individual cases of spontaneous diabetes in NHPs
The progression of diabetes can vary among human patients and also in diseased NHPs. To show the general characteristics of diabetic progression in NHPs, Figure 3 summarized the changes of body weight, HbA1c, glucose, insulin and lipids in our housed diabetic NHPs. The average body weight decreased gradually following the ages when the diabetic animals were getting old (Figure 3A). Along with the decrease in body weight, serum insulin level also decreased progressively when the animals got older (Figure 3B). In contrast, blood HbA1c and serum glucose levels gradually increased during the aging process (Figures 3A and 3B). Blood TG and TC kept on staying at high levels with some obvious variabilities during the disease process (Figure 3C). The average blood LDL levels in the diabetic NHPs had some moderate increase during the early ages and then increased markedly at their late ages (Figure 3D). However, the blood HDL levels were in a normal range with much less variations during the disease process (Figure 3D). It is interesting that the diabetic monkeys with ages over 24 years old were found to be female only in this study. Such phenomenon is similar to in humans that women have a longer lifespan than men [38].
Figure 3: Progressive changes of body weight ( ) and HbA1c (
) and HbA1c ( ) shown in panel A, glucose (
) shown in panel A, glucose ( ) and insulin (
) and insulin ( ) in panel B, TG (
) in panel B, TG ( ) and TC (
) and TC (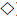 ) in
panel C, and HDL (
) in
panel C, and HDL (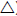 ) and LDL (
) and LDL (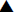 ) in panel D in diabetic NHPs aged from 10 to 28 years. Each data point shows the mean of the results collected
from various diabetic animals (n=5 to 28).
) in panel D in diabetic NHPs aged from 10 to 28 years. Each data point shows the mean of the results collected
from various diabetic animals (n=5 to 28).
To further depict the variations of progressive hyperglycemia in NHPs, the changes of body weight, serum glucose, HbA1c and insulin were presented from the selective individual monkeys with normoglycemia (monkey B07, identification #), mild hyperglycemia (approximately 100 mg/dL, O07), and severe hyperglycemia (250 mg/dL, U04) during past 5 to 10 years after they were housed in our animal facility. The normoglycemic one (B07) was healthy during past over 5 years (from age 13 to nearly 19 years old) with a moderate increase in body weight and minor changes in serum glucose, insulin and HbA1c levels (Figure 4, left panel). The moderate hyperglycemic animal (O07) showed stable body weight and blood HbA1c during the past 10 years (from age 14 to 24 years old) with a minor increase in serum glucose and insulin (decreased lately) levels (Figure 4, middle panel). The more severe hyperglycemic animal (U04, female) showed stable body weight, serum glucose and HbA1c during the past 5 years (ages from 11 to 15.6 years old) with a minor decrease in serum insulin (Figure 4, left panel). These results indicate that not only normal healthy monkeys, but also moderate and severe hyperglycemic animals could survive in our animal facility for many years (5-10 or longer) without dramatic changes on their diabetic conditions.
Figure 4: Changes of body weight ( ), serum glucose (
), serum glucose (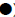 ), blood HbA1c (
), blood HbA1c ( ) and serum insulin (
) and serum insulin ( ) in individual monkeys following the time course
after they were housed in our animal facility. Normoglycemia (monkey ID-B07) and hyperglycemia (moderate, ID-O07 and severe, ID-U04) were selected
for the representative individuals to show their progressive changes following their ages from 11 to 24 years old.
) in individual monkeys following the time course
after they were housed in our animal facility. Normoglycemia (monkey ID-B07) and hyperglycemia (moderate, ID-O07 and severe, ID-U04) were selected
for the representative individuals to show their progressive changes following their ages from 11 to 24 years old.
In contrast, some monkeys showed progressively changes in their diabetic conditions. The serum glucose level and HbA1c of animal O01 were approximately 100 mg/dL and 5%, respectively, at ages before 19 years old (Figure 5). The serum glucose levels gradually increased to nearly 500 mg/dL and HbA1c raised to 11% or higher at age 24. In the meantime, body weight and serum insulin were decreased by more than 20% (Figure 5, O01). The progressive increases in serum glucose and HbA1c with the decreases in insulin and body weight were observed in monkey L09 during his ages of 14-19 years old (Figure 5, L09). Animal B09 showed a slow increase in serum glucose level, but remained relatively stable body weight, insulin and HbA1c during past 6 years in our animal facility (Figure 5, B09). However, monkey B05 showed hyperglycemia at age 13 with high insulin, overweight (15 kg) and mild HbA1c (6%). Starting from age 15, the animal showed dramatic increases in serum glucose and HbA1c with a big increase in insulin with markedly reduction of body weight. After age 16, serum glucose and HbA1c remained at high levels, but insulin decreased to a new low level. Body weight continuously decreased by a total of nearly 50% during the past 7 years (from 13 to 20 years old, Figure 5, B05). Our data demonstrate that progressions of diabetes in individual monkeys vary, which is similar to in humans as individual patients need to be treated specifically according to patients’ various conditions.
Figure 5: Progressive changes of body weight ( ), serum glucose (
), serum glucose ( ), blood HbA1c (
), blood HbA1c (  ) and serum insulin (
) and serum insulin ( ) in individual diabetic monkeys following
the time course after they were housed in our animal facility. Representative monkeys (ID-O01, L09, B09 and B05) during their ages from 13 to 24 years
old showed progressively worsening on their serum glucose and insulin levels, as well as blood HbA1c. In addition, their body weights continuously
decreased when their serum glucose levels progressively arised.
) in individual diabetic monkeys following
the time course after they were housed in our animal facility. Representative monkeys (ID-O01, L09, B09 and B05) during their ages from 13 to 24 years
old showed progressively worsening on their serum glucose and insulin levels, as well as blood HbA1c. In addition, their body weights continuously
decreased when their serum glucose levels progressively arised.
In order to learn the correlation between diabetes and dyslipidemia,
changes of body weight, serum glucose, insulin, HbA1c, TG, TC,
HDL and LDL in diabetic animals were collected and analyzed.
Monkey Q07 (Figure 6, left panels) showed the increase in serum
glucose levels ( ) accompanied with increases in TG (
) accompanied with increases in TG ( ), LDL (
), LDL ( )
and HbA1c (
)
and HbA1c ( ), and with some decrease in HDL (
), and with some decrease in HDL ( ) and no much
changes on TC (
) and no much
changes on TC ( ), body weight (
), body weight (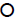 ) and insulin (
) and insulin (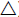 ). The similar
manifestations of diabetic progression were observed in monkey
R07 during past 5 years (Figure 6, right panels). Serum glucose (
). The similar
manifestations of diabetic progression were observed in monkey
R07 during past 5 years (Figure 6, right panels). Serum glucose ( ) progressively increased accompanied with correspondingly arising
in TG (
) progressively increased accompanied with correspondingly arising
in TG (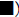 ), LDL (
), LDL ( ) and HbA1c (
) and HbA1c (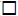 ). However, body weight
(
). However, body weight
(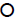 ), insulin (
), insulin (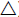 ) and HDL (
) and HDL ( ) were correspondingly reduced
with minor changes on TC (
) were correspondingly reduced
with minor changes on TC (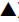 ). Our data indicate that diabetic
procession in NHPs accompanies with dyslipidemia and body
weigh changes, which could be similar to the clinical progression
in diabetic patients even though our diabetic monkeys enrolled in
this study were not with antidiabetic therapy. Therefore, NHPs
could be reliably used in preclinical studies as NHPs show
high similarities to human genomics, physiology and disease
process [39-42]. Evidently, the results derived from NHP studies
have paralleled the results of later studies performed in humans
[43,44], which reinforces the utility of NHPs as an excellent
preclinical animal model for investigating the physiology of human
metabolism and the pathophysiology of metabolic dysfunction.
). Our data indicate that diabetic
procession in NHPs accompanies with dyslipidemia and body
weigh changes, which could be similar to the clinical progression
in diabetic patients even though our diabetic monkeys enrolled in
this study were not with antidiabetic therapy. Therefore, NHPs
could be reliably used in preclinical studies as NHPs show
high similarities to human genomics, physiology and disease
process [39-42]. Evidently, the results derived from NHP studies
have paralleled the results of later studies performed in humans
[43,44], which reinforces the utility of NHPs as an excellent
preclinical animal model for investigating the physiology of human
metabolism and the pathophysiology of metabolic dysfunction.
Figure 6: Selected individual diabetic monkeys represented the correlated changes of body weight (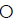 ), serum glucose (
), serum glucose ( ), blood HbA1c (
), blood HbA1c ( ) and serum
insulin (
) and serum
insulin ( ) with serum lipids of TG (
) with serum lipids of TG ( ), TC (
), TC ( ), HDL (
), HDL ( ) and LDL (
) and LDL (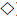 ) during the time being housed in our animal facility. Monkey Q07 and R07
showed progressively increases in glucose, HbA1c, TG and LDL. However, their HDLs decreased and R07 body weight and insulin also reduced.
) during the time being housed in our animal facility. Monkey Q07 and R07
showed progressively increases in glucose, HbA1c, TG and LDL. However, their HDLs decreased and R07 body weight and insulin also reduced.
Drug-induced hyperglycemia in NHPs is also broadly used in diabetic research, especially for islet or beta cell transplantation. Streptozocin (STZ), a naturally occurring glucosamine-nitrosourea compound, has been used for diabetogenic induction in NHPs due to its high toxicity to pancreatic beta cells [45]. The diabetogenic effects of STZ by multiple low doses or by single high dose in normoglycemic NHPs were investigated [46]. Each monkey in the 1st group (n=6) was intravenously administered with 7.5 to 15 mg/kg STZ once every 2 to 4 weeks until successful induction of hyperglycemia or until the end of this 28-week study. In the 2nd group (n=7) each monkey was intravenously injected with 35 mg/ kg STZ once only. Compared with STZ multiple low doses, single high dose caused much severe insulin depletion, similar to Type I diabetes. Among them one animal died on the 2nd day after STZ high dose [46]. Beta-cell sensitivity to STZ toxicity varied obviously among individual monkeys, some highly sensitive and some almost no response. Therefore, extra caution may need to be taken to avoid incidental death if single high dose is administered. The successful rate of diabetogenic effects was approximately 50% with multiple low doses of 15 mg/kg and 83% after one high single dose 35 mg/ kg with great loss of beta cells in islets (Figure 7). Litwak et al. found 30 mg/kg STZ did not reliably cause diabetes and only 35% became mildly hyperglycemic in cynomolgus monkeys [47]. Fifty mg/kg STZ reached 80% hyperglycemia in treated cynomolgus monkeys [48]. Using 55 mg/kg STZ induced 100% diabetogenic effects for up to 1 year without any evidence of regeneration of the beta-cells in cynomolgus monkeys [49]. The diabetogenic effects of STZ were also examined in rhesus monkeys and found 71% animals treated with 45 mg/kg and 100% animals received 60 mg/kg developed hyperglycemia [50]. These results indicate that nonhuman primates show their various responses to STZ diabetogenic induction.
Figure 7: Successful induction of hyperglycemia with multiple low doses (15 mg/kg*x) or single high dose (35 mg/kg) of STZ in cynomolgus or rhesus monkeys. Each monkey in cynomolgus group (n=6) was intravenously administered with multiple doses of 15 mg/kg STZ once every 2 to 4 weeks until successful induction of hyperglycemia or until the end of study. Each monkey in rhesus group was intravenously injected with 35 mg/kg STZ once. Increase in serum glucose (%, panel A) and the successful ratio of hyperglycemia is shown in panel B. Immunohistochemical staining against insulin in islets observed from paraffin-sectioned pancreatic slides (20x) in the monkeys with normoglycemia (C, green area) and with hyperglycemia by >300 mg/ dL constantly (D, scattered green areas) after STZ treatment.
The underline mechanism of such obvious variability in NHPs responded to STZ is not fully delineated. Diabetogenic dose of STZ may relate to the animal age, body weight, route of administration, and nutritional status [51-53]. In addition, the individual genetic, health and physiological differences, such as receptor expression levels, may also contribute to the wide variance in STZ doses among experimental animals. Another possibility of these discrepancies could be beta-cells in some monkeys are more resistant to STZ toxic effect. For example, insulin producing cells that do not express GLUT2 (glucose transporter 2) in the plasma membrane are resistant to STZ toxicity and only become vulnerable to STZ toxicity after expression of GLUT2 protein in the plasma membrane [54]. Therefore, to successfully establish STZ-induced diabetic model in NHPs, an optimal dose or feasible approach is necessary. Such dose or approach can induce irreversible and stable diabetes (more similar to T2DM) with much less adverse effects. Our data provide some insights on the model induction in NHPs, because approximately 50% monkeys treated with multiple low doses of STZ were hyperglycemic without any observable side effects and with no need of exogenous insulin therapy (Figure 7). Our data demonstrate that single high dose of STZ resulted in more severe diabetogenic effects (similar to type I) than multiple low doses (similar to Type II). Single high dose of STZ was accompanied more serious side effects, including insulin dependence, liver damage, kidney impairment and animal death. These results may help researchers to understand the diabetogenic process and variability of STZ induction in NHPs and to choose a severe or moderate hyperglycemic model for their research need.
More monkeys are enrolled in biomedical research in recent years and reached a record number according to one 2018 report [55]. Significant advantages of NHP models for drug and new therapy development include their close genetic identity and physiological similarity to humans, especially in studies involving recombinant biologics targeting human receptors or proteins. Another advantage of studies in NHPs compared with in humans is that diet and pharmacological interventions can be well controlled, whereas compliance with dietary and drug treatment regimens is generally quite poor in free-living human subjects. In fact, in dietary studies in NHPs, compliance with the dietary regimen can be knownMore monkeys are enrolled in biomedical research in recent years and reached a record number according to one 2018 report [55]. Significant advantages of NHP models for drug and new therapy development include their close genetic identity and physiological similarity to humans, especially in studies involving recombinant biologics targeting human receptors or proteins. Another advantage of studies in NHPs compared with in humans is that diet and pharmacological interventions can be well controlled, whereas compliance with dietary and drug treatment regimens is generally quite poor in free-living human subjects. In fact, in dietary studies in NHPs, compliance with the dietary regimen can be known with near certainty. Compared with extremely strict limitations in humans, biopsies of a variety of tissues including adipose, muscle, liver and kidney can be performed in NHP studies, particularly with minimally invasive approaches (e.g., echography-guided or laparoscopic biopsy). In addition, tissues samples, such as central neuronal tissues from critical areas or from other life-organs, cannot be obtained in humans, but can be collected at the time of necropsy in NHP studies designated to have a terminal endpoint. Furthermore, compared with rodents or other small animals, relatively large blood volume possibly being sampled in NHPs is also critical for some studies which require frequent bleedings with enough amount for multiple assays.
The main disadvantages of using NHPs versus other animal models can be the limited resources of animals and high expenses for maintaining colonies and performing experiments. Of course, compared with similar clinical trials, NHP studies are far less expensive [56]. However, NHP studies still can lead to greater and more rapid advances in the evaluation and translation of novel therapies. In addition, specific facilities and technical expertise are extremely important for running successful NHP studies.
The global prevalence of diabetes requires extensive investigation on understanding its mechanism and developing new therapy, which can be markedly tardy by lack of critical animal models. In this article the diabetic models of NHPs are introduced and characteristic data are presented. The general metabolic manifestation of the monkeys with naturally occurring diabetes was significantly older, compared with non-diabetic ones. Their ages correlated well with their blood glucose and HbA1c levels, but did not correlate well with blood insulin levels. In addition, their body weights correlated well with their blood levels of glucose, HbA1c, insulin and C-peptide. Our data also show the progression of diabetes in the NHPs during past 5 to 10 years. The results of ivGTT and oGTT demonstrate the development of insulin resistance in diabetic NHPs. Although none of preclinical models can completely capture the complexity of human dysmetabolic syndrome, the naturally occurring diabetes in NHPs is most likely the closest preclinical model to human diabetes, such as loss of blood glucose control, complications and dyslipidemia.
Research involving human participants and/or animals
This study did not involve humans, but was conducted in monkeys. The study protocol and experimental procedures used in this study were approved by the IACUC of Crown Bioscience Inc., which includes member from outside of the company. The approval numbers are AN-1308-016-19 and AN-1507-004.
Conflict of interests
The authors are employee of Crown Bioscience Inc. The authors declare no conflict of interest in this study.
Informed consent
The authors have read and approved the manuscript for submission.
Their consents are available if requested.
Availability of data and material
All the materials and relevant raw data supporting our findings can be found in Tables and Figures in the manuscript and are freely available to readers or scientists wishing to use them for noncommercial purposes.
Funding
This study was supported by the internal research fund from Crown Bioscience, Inc.
Authors' contributions
Y-F Xiao and Y (Jim) Wang participated in study design, data collection and analysis, as well as figure and manuscript preparation.
We are very grateful to our department employees who participated the experiments and data collections and also to our animal center employees for their professional care of the animals and excellent technical assistances during the study.
Citation: Xiao YF, Wang YJ (2019) Dysglycemia and Dyslipidemia Models in Nonhuman Primates: Part IV. Pancreatic Beta Cell Dysfunction and Diabetes Progression. J Diabetes Metab. 10:832. doi: 10.35248/2155-6156.19.10.832
Received: 16-Aug-2019 Published: 10-Sep-2019, DOI: 10.35248/2155-6156.19.10.832
Copyright: © 2019 Xiao YF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.