Mini Review - (2015) Volume 3, Issue 4
Lignans are polyphenols spread in a wide variety of plant-based foods, including seeds, whole grains, legumes, fruit, and vegetables. When consumed, lignan precursors are converted to the enterolignans, enterodiol and enterolactone, by bacteria that normally colonize the human intestine. Lignans have attracted considerable attention due to their pharmacological and biological activities. This review article is a survey on the molecular structure of about 276 new naturally occurring lignans obtained from different plant families. We classified seven main types according to their structural features, and provided a number of review reports published recently about lignans. A tabular compilation of the molecular structures of known lignans is presented at the end of this survey report. A total of 111 references were considered for this survey report.
Keywords: Lignans; Molecular structures; Bioactivities; Survey report
For centuries, the structural studies of natural products served as the principle driving force for the discovery of new chemical reactivity. The fact that natural products are an excellent reservoir of biologically active compounds. For long time, extracts from natural products have been a main source of folk medicines, and even today, many cultures still employ them directly for medicinal purposes. Natural product chemistry turned to an interdisciplinary science, where the success of a chemist would only be possible in close collaboration with biologists, pharmacologists, and chemists. Thus many novel biological activities could only have emerged from the natural products arena.
Among the classes of identified natural products, lignans, one of the largest families, have been studied intensively for their diverse structures and variety of biological activities. Lignans are widely distributed in nature, occurring in hundreds of plants all over the world. Many lignane structures have interesting biological, pharmacological, or medicinal activities, including inhibition of carcinogenesis and induction of differentiation in leukemia or teratocarcinoma cells. Lignans are structurally diverse organic compounds, characterized by a basic backbone modified in multiple ways, allowing the formation of more than 7000 naturally occurring lignan varieties, particularly with approximately 50 new lignans identified each year. The amount of research effort performed towards lignans has been striking increased dramatically since 1940. For example, over the periods 1940-1960 and 1960-present, the average number of refereed publications describing research involving lignans as a major component (within abstract, title, and keywords; estimated using Scopus search engine) was 11 and 7016, respectively. It is interesting to note that the publications concerned with lignans observed between 2000 (189 documents) and 2015 (420 documents) coincides with the most recent discoveries on occurrence, extraction, isolation, purification, classification, biosynthesis, biogenesis, and applications of lignans.
Lignans, a vast, abundant and diverse group of secondary metabolites distributed in higher plants generally derivatives of phenyl propanoids created naturally via peroxidases and laccases enzymes. They induce a structural diversity and mainly interesting bioactivities, thus, the new research has been dedicated to the development of effective novel methodologies for biosynthesis of lignans, analogs and hybrids [1-3]. A recent studies presents a variety of synthetic and biosynthetic methods and mechanistic schemes which mimic the most common and various lignans and norlignans organic skeletal (Scheme 1) [4,5]. Another recent work shows phytochemical and pharmacological properties of the isolated, purified anticancer lignans from Anthriscus sylvestris (L.) Hoffm. herbaceous plant [6].
The anti-cancerous, estrogen like, antiasthma anti-inflammation, antioxidant, antimicrobial, insecticidal, antihypertensive, hypocholesterolemic activities and hepatoprotective biological activities of dibenzylbutyro-lactone subgroup of lignans were reported recently [7]. A number of various key multi-step racemic and enantiospecific synthetic strategies of plant and mammalian epoxy and dibenzocyclooctadiene lignans and such as Diels-Alder reactions, Stobbe condensations, transition metal-mediated, Michael additions, alkylations, intermolecular/intramolecular aryl-aryl oxidative couplings, nitrile oxide cycloadditions, radical cyclisations, dianion and oxidative couplings were reviewed [8-11]. A group of clinically important antioxidant lignans extracted from lariciresinol, pinoresinol, odophyllum peltatum, linum, hyptis, anthriscus, juniperus, dysosma, medioresinol, syringaresinol, arctigenin and sesamin wild species were found to play a key role in protection against several hormones related diseases and breast, colon, and prostate cancer therapy [12-15]. The different possible mechanistic elucidations for the detected various bioactivities such as angiogenesis, anti-oxidation and gene suppression [13] were evaluated and studied.
The extraction, isolation, purification, biosynthesis, structural and spectral characteristics of aryltetralin lactone lignans [16] from Euphorbiaceae, Berberidaceae, Verbenaceae, Burseraceae, Acanthaceae, Taxus baccata L. [17] plants and coumarino, flavono, and stilbeno nonconventional lignans [18] were reported recently, as well evaluated for their anti-inflammatory, anti-nociceptive, anti-ulcerogenic, antimicrobial, functional nutrient additives, chemo-ecological, cytotoxic, and antioxidant as well as acetylcholinesterase, butyrylcholinesterase and lipoxygenase inhibitory activities [17,19-30]. Also, recent study showed that the physiologically significant concentrations of enterolignan-producing bacteria were leading to "in vitro" and "in vivo" activation of oestrogen receptors [13]. The metabolism, absorption and excretion of phyto-oestrogen dietary lignans were also reported [19]. Table 1 shows the molecular structures of more than 270 known lignans.
| Lignan Name | Molecular Structure | Ref. | Lignan Name | Molecular Structure | Ref. | |
|---|---|---|---|---|---|---|
| Schizandrin | 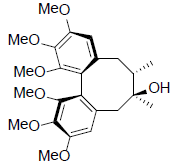 |
[31] | Gomisin A | 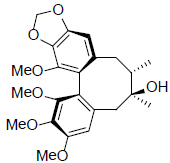 |
[32] | |
| Deoxyschizandrin | 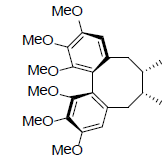 |
[33] | Gomisin B R=OAng Gomisin C R=OBz |
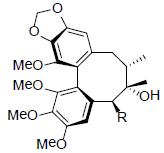 |
[34] | |
| γ-Schizandrin | 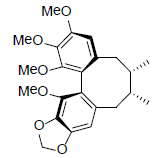 |
[35] | Gomisin D | 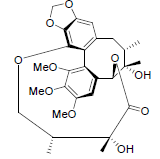 |
[36] | |
| Isoschizandrin | 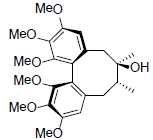 |
[37] | Gomisin F R=OAng Gomisin G R=OBz |
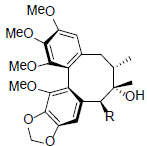 |
[38] | |
| Schisandrin C | 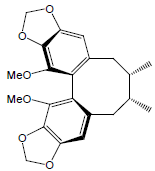 |
[39] | Gomisin P R=OH Benzoylgomisin P R=OBz Tigloylgomisin P R=OTig Angeloylgomisin P R=OAng |
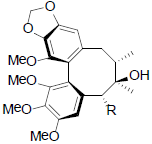 |
[40-43] | |
| Gomisin F R=OAng Gomisin G R=OBz |
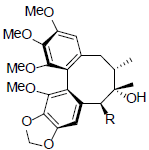 |
[44] | Gomisin Q | 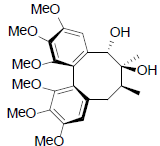 |
[45] | |
| Gomisin H | 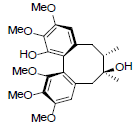 |
[46] | Angeloylgomisin Q R=OAng Benzoylgomisin Q R=OBz |
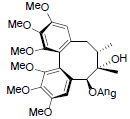 |
[41,46] | |
| Angeloylgomisin H R=OAng Tigloylgomisin H R=OTig Benzoylgomisin H R=OBz |
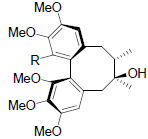 |
[46] | Gomisin R | 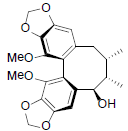 |
[47] | |
| Gomisin J | 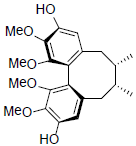 |
[46] | Gomisin S | 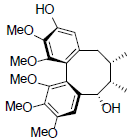 |
[48] | |
| (-)-Gomisin K1 | 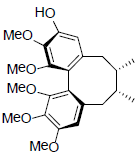 |
[49] | Gomisin T | 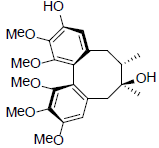 |
[48] | |
| (+)-Gomisin K2 R1=OH, R2=OMe (+)-Gomisin K3 R1=OMe, R2=OH |
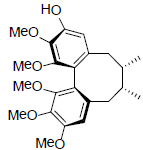 |
[49] | Benzoylgomisin U | 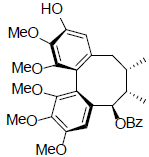 |
[50] | |
| (-)-Gomisin L1 R1=OMe, R2=OH (-)-Gomisin L2 R1=OH, R2=OMe |
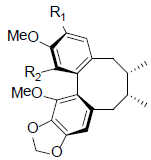 |
[47] | Acetyl binankadsurin A R=OAc Angeloyl-binankadsurinA R=OAng Caproyl-biannkadsurin A R=OCap |
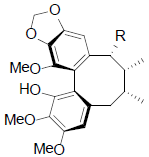 |
[51] | |
| (±)-Gomisin M1 R1=OH, R2=OMe (+)-Gomisin M2 R1=OMe, R2=OH |
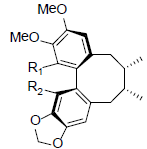 |
[47] | Heteroclitin A R=OIsval Heteroclitin B R=OAng Heteroclitin C R=OTig |
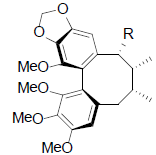 |
[52] | |
| Gomisin N | 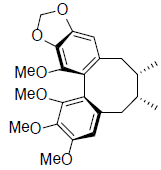 |
[40] | Heteroclitin G | 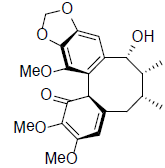 |
[52] | |
| Gomisin O R1=OH, R2=H Isogomisin O R1=H, R2=OH Angeloylgomisin O R1=OAng, R2=H |
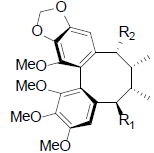 |
[39,40,47] | Tigloylgomisin O | 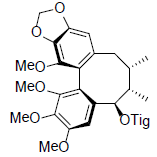 |
[50] | |
| Angeloylisogomisin O R=OAng Benzoylisogomisin O R=OBz |
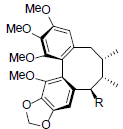 |
[40] | Taiwankadsurin B | 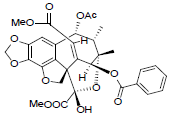 |
[53] | |
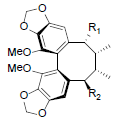
| Schisantherin L R1=OH, R2=OAng Schisantherin M R1=OTig, R2=OAng Schisantherin N R1=R2=OAng Acetylschisantherin L R1=OAc, R2=OAng |
 |
[54] | Interiotherin A | 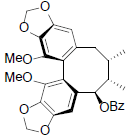 |
[55] | |
| Schisantherin O | 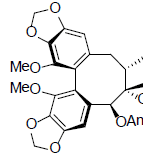 |
[54] | Interiotherin B | 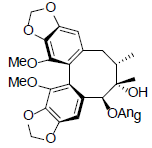 |
[55] | |
| Kadsulignan L R1+R2=OCH2O, R3=OMe Kadsulignan M R1+R2=OCH2O, R3=OH Kadsulignan N R1=R2=R3=OMe |
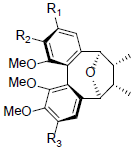 |
[56] | Angustifolin A R=OBz Angustifolin B R=OAc Angustifolin C R=OH |
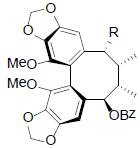 |
[56] | |
| Schisantherin P | 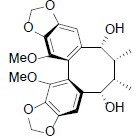 |
[56] | Angeloylbinankadsurin B R=OAng Acetylbinankadsurin B R=OAc |
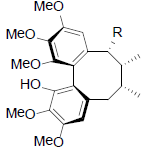 |
[57] | |
| Schisantherin Q | 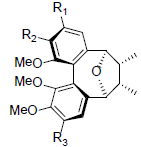 |
[56] | Heteroclitin N | 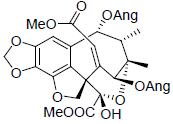 |
[58] | |
| Schizanrin A | 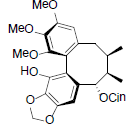 |
[59] | Longipedunin C | 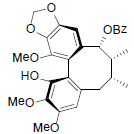 |
[60] | |
| Kadsumarin A | 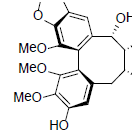 |
[59] | Kadsuralignan A | 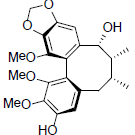 |
[61] | |
| Ananosin A | 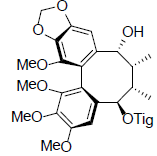 |
[62] | Kadsuralignan B | 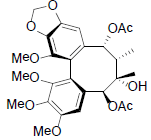 |
[61] | |
| Schizarin B R=OIsoval Schizarin C R=OCap Schizarin D R=OAc Schizarin E R=OBz |
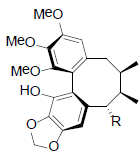 |
[63] | Schizanrin K | 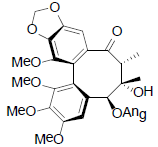 |
[64] | |
| Interiotherin C | 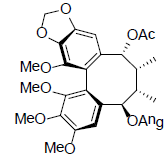 |
[65] | Schizanrin L | 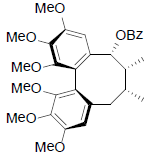 |
[64] | |
| Schizanrin F R1=OMe, R2=OBz R1=OH, R2=OAng |
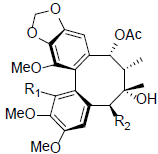 |
[66] | Schizanrin M | 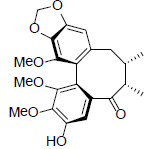 |
[64] | |
| Schizanrin H | 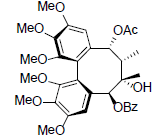 |
[66] | Renchangianin D | 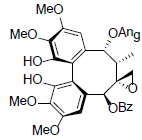 |
[67] | |
| Renchangianin A R=OAc Renchangianin B R=OAng |
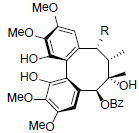 |
[67] | Rubriflorin A | 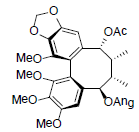 |
[68] | |
| Renchangianin C | 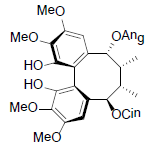 |
[69] | Rubriflorin B | 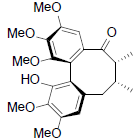 |
[68] | |
| Yunnankadsurin A | 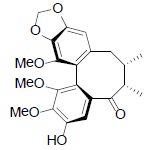 |
[70] | Schizanrin N Schizanrin N | 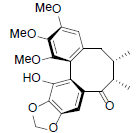 |
[64] | |
| Yunnankadsurin B | 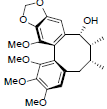 |
[70] | Heteroclitalignan A R1=OBz, R2=OAc, R3=OH Heteroclitalignan B R1=OPro, R2=OAng, R3=OMe Heteroclitalignan D R1=OBz, R2=OAc, R3=OMe |
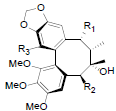 |
[71] | |
| Schizanrin I R1=OBz, R2=OBz Schizanrin J R1=OAng, R2=OAng |
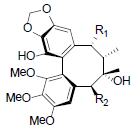 |
[64] | Longipedunin A R=OCin Longipedunin B R=OProp |
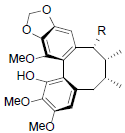 |
[60] | |
| Propinquanin A R1=OMe, R2=OTig Propinquanin B R1=OH, R2=OBz |
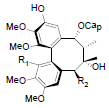 |
[72] | Propinquanin D | 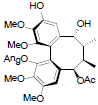 |
[72] | |
| Propinquanin C | 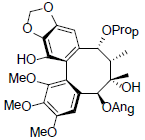 |
[72] | Propinquanin E | 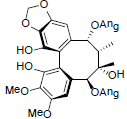 |
[72] | |
| Propinquanin F | 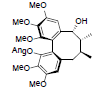 |
[72] | Schisandrene | 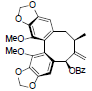 |
[73] | |
| Kadsuphilin A R=OMe 1-Demethylkadsuphilin A R=OH |
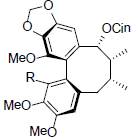 |
[74] | Kadsuphilin B | 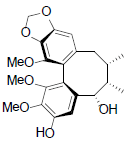 |
[74] | |
| 6-Epi-gomisin | 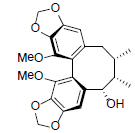 |
[74] | Kadsuphilin C R1+R2=OCH2O Kadsuphilin E R1=R2=OMe |
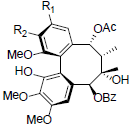 |
[75] | |
| Rubrisandrin A R1=OH, R2=OMe Rubrisandrin B R1=OMe, R2=OH |
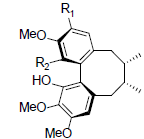 |
[76] | Kadsuphilin D R=OAc Kadsuphilin F R=OBz |
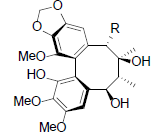 |
[75] | |
| Kadsuphilol A | 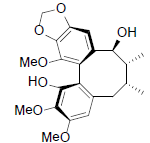 |
[75] | Kadsuphilol C | 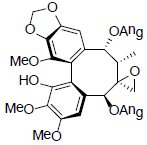 |
[75] | |
| Kadsuphilol B | 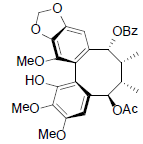 |
[75] | Kadsuphilol D | 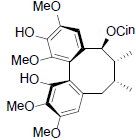 |
[75] | |
| Angeloyl-(+)-gomisin K3 | 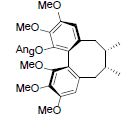 |
[77] | Kadangustin B | 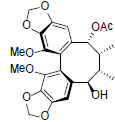 |
[78] | |
| Methylisogomisin O | 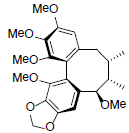 |
[79] | Kadangustin C | 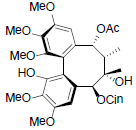 |
[78] | |
| Kadsurindutin A R=OAc Kadsurindutin B R=OH |
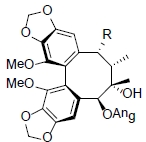 |
[79] | Neglschisandrin A | 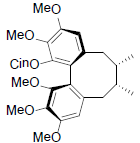 |
[80] | |
| Kadsuralignan I R=OAng Kadsuralignan J R=OIsoval Kadsuralignan K R=OBz |
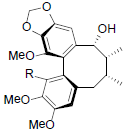 |
[81] | Neglschisandrin B | 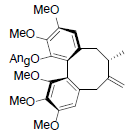 |
[82] | |
| Kadsuphilin G R1=OAc, R2=H Kadsuphilin H R1=OAng, R2=OH |
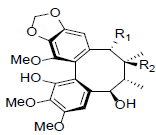 |
[82] | Neglschisandrin C | 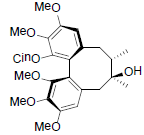 |
[82] | |
| Kadsuphilin I R1=OH, R2=OBz Kadsuphilin K R1=OMe, R2=OH |
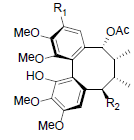 |
[82] | Neglschisandrin D | 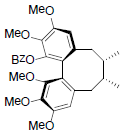 |
[82] | |
| Kadangustin A R=OH Kadangustin D R=OCin Kadangustin E R=OBz Kadangustin F R=OAng Kadangustin G R=OTig |
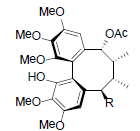 |
[78] | Heteroclitin P R1=OBz, R2=OAng Heteroclitin Q R1=OAc, R2=OBz |
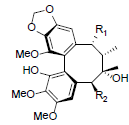 |
[83] | |
| Kadoblongifolin A R1=OH, R2=OMe Kadoblongifolin B R1=OMe, R2=OH Kadoblongifolin C R1=OMe, R2=OMe |
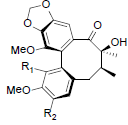 |
[84] | Tiegusanin A R1=OMe, R2=OIsoval Tiegusanin B R1=OMe, R2=OIsobut Tiegusanin C R1=OBz, R2=OMe |
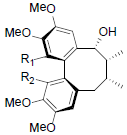 |
[85] | |
| Tiegusanin K | 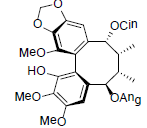 |
[85] | Kadsuphilol I | 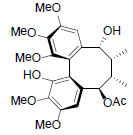 |
[86] | |
| Tiegusanin L | 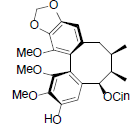 |
[85] | Kadsuphilol J | 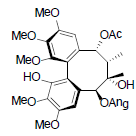 |
[86] | |
| Tiegusanin M | 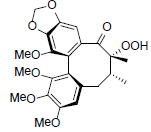 |
[85] | Taiwankadsurin A R1=OBz, R2=OAc Taiwankadsurin C R1=OAc, R2=OBz |
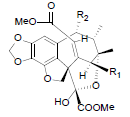 |
[53] | |
| Kadsuphilol K | 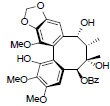 |
[86] | Arisanschinin F | 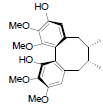 |
[86] | |
| Kadsuphilol L | 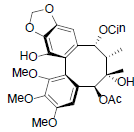 |
[86] | Arisanschinin G | 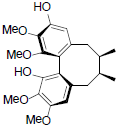 |
[86] | |
| Arisanschinin H | 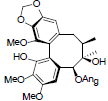 |
[86] | Arisanschinin I | 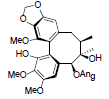 |
[86] | |
| Arisanschinin K R=OBz Arisanschinin L R=OAng |
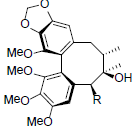 |
[86] | Arisanschinin J | 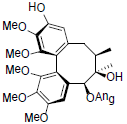 |
[86] | |
| Schisanwilsonin A | 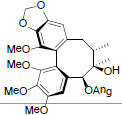 |
[87] | Schisanwilsonin C | 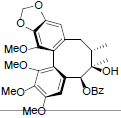 |
[87] | |
| Schisanwilsonin B | 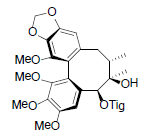 |
[87] | Schisanwilsonin D | 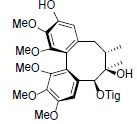 |
[87] | |
| Schilancifolignan A | 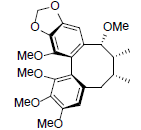 |
[88] | Schilancifolignan B | 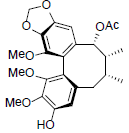 |
[88] | |
| Schilancifolignan C | 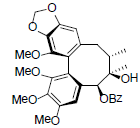 |
[88] | Taiwanschirin A R=OIsoval Taiwanschirin B R=OAc Taiwanschirin C R=OBz |
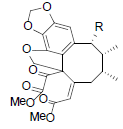 |
[59] | |
| Heteroclitin D R1=OAng, R2=H Heteroclitin E R1=OAng, R2=OH |
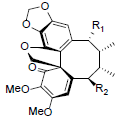 |
[89] | Interiotherin D | 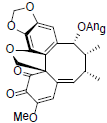 |
[65] | |
| Schiarisanrin A R=Isoval Schiarisanrin B R=OAc Schiarisanrin C R=OBz Schiarisanrin D R=OCin |
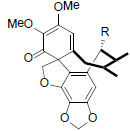 |
[90] | Schiarisanrin E | 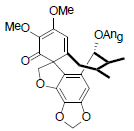 |
[64] | |
| Heteroclitalignan C | 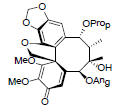 |
[17] | Kadsuphilol H | 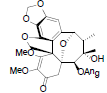 |
[75] | |
| Kadsuphilol E R1=OAng, R2=OBz Kadsuphilol F R1=OBz, R2=OAng |
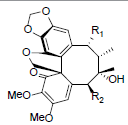 |
[75] | Kadsuphilin L | 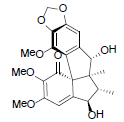 |
[82] | |
| Kadsuphilol G | 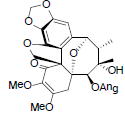 |
[75] | Arisantetralone C | 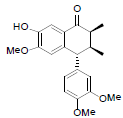 |
[86] | |
| Kadsuphilin M | 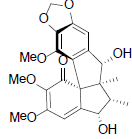 |
[82] | Kadsutherin D | 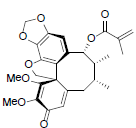 |
[91] | |
| Heteroclitin I | 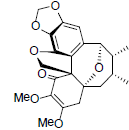 |
[58,91] | Heteroclitin O | 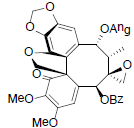 |
[93] | |
| Heteroclitin J | 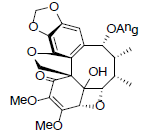 |
[85] | Kadsuphilol M | 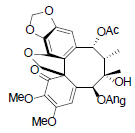 |
[86] | |
| meso-Dihydroguaiaretic acid | 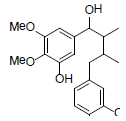 |
[92] | Kadangustin H | 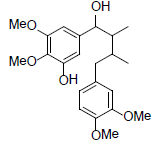 |
[78] | |
| Pre-gomisin | 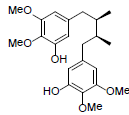 |
[40] | Kadangustin I | 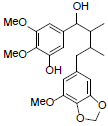 |
[78] | |
| Kadsuphilin J | 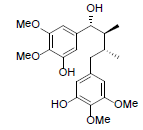 |
[78] | Heteroclitin F | 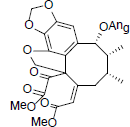 |
[52] | |
| Kadangustin J R=OMe Kadangustin K R=OH |
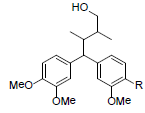 |
[78] | Henricine B | 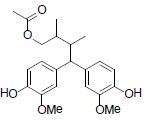 |
[93] | |
| Tiegusanin N | 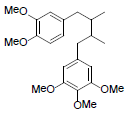 |
[85] | Arisantetralone D | 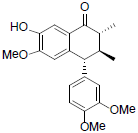 |
[86] | |
| Enshicine | 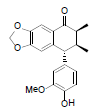 |
[94] | Kadsuralignan C | 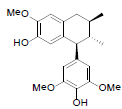 |
[61] | |
| Desoxyenshicine R1=R2=-CH2- Desoxydemethylenshicine R1=R2=H |
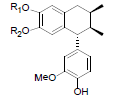 |
[94] | Kadsuralignan H | 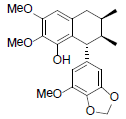 |
[81] | |
| (8R,8'R)-8-Hydroxy-3,4- dimethoxy-3',4'-methylenedioxy- 7-oxolignan |
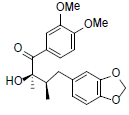 |
[85] | Arisantetralone A R1=OMe, R2=OH Arisantetralone B R1=OH, R2=OMe |
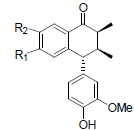 |
[86] | |
| (7'R, 8'S)-3,4-Methylenedioxy-3', 4'-dimethoxy-7,8–seco–7,7'–epoxylignan-7,8-dione |
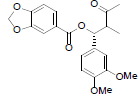 |
[85] | ||||
 |
||||||
Table 1: Molecular structures of 267 Known lignan.
A number of review articles reported between 1980 and 2000 about asymmetric synthesis approaches of lignans and stereo controlled syntheses techniques of unsymmetrically furofuran lignans, and on plant natural lignans, neolignans and their cancer chemoprevention, cardiovascular, antiviral, food and biotechnological applications [95-111].
This work was financially supported by King Khalid University and King Abdulaziz City for Science and Technology (KACST), Saudi Arabia.