Research Article - (2016) Volume 4, Issue 3
Iron nitrate supported hexagonal mesoporous (Fe-HMS) catalyst were successfully synthesized for Fenton-like oxidative degradation of Reactive Green-19 (RG19). Effects of pH, light intensity, catalyst amount, dye concentration, sensitizers, etc., were studied to optimize conditions for enhanced photo-catalytic activity. SEM, EDX, BET and UV–Vis (diffused reflectance mode) techniques were used to revealed the Fe-HMS physico-chemical properties. Kinetic study RG-19 treatment process at a reaction temperature 40°C, pH 3, 3 mgL-1 of Fe-HMS, 50 μL of H2O2, and 100 mgL-1 of dye were successfully carried out. The Fenton-like process correlation coefficient favors the first order kinetic model. COD and metal leaching analysis were done and results revealed that they were below DOE standard for effluent. Therefore, the treated water can safely discharge to the environment. The Fe-HMS catalyst reusability proved that it could be reused and has stability in reaction more than four cycles.
Keywords: Fenton-like catalysts; Fe-hms; Reactive green-19; Degradation process; Physic-chemical characterizes; Kinetic and reusability studies
Based on the concern with regards to the environmental impact, the word is directed towards fulfilling the demands for safe wastewater disposal [1]. Treatment of dye pollutants must focus on reducing the concentration of the original dye compounds and their degradation products in wastewater. Related issues could include excessive production of sludge and required space for the physic-chemical treatment process, as well as longer detention time required in biological treatment process [2].
Mostly the dye pollutants produced by the textile industries show environmental and health issues [3]. These forms of pollutants are usually toxic and have to be treated before discharge [4]. Most of the methods used in the treatment of organic pollutants such as coagulation membrane, filtration, and biological treatment might also face other problems such as membrane fouling, formation of sludge, and incomplete mineralization [5]. Also, homogenous Fenton process could have certain drawbacks, as the Fe ion itself leads to several disadvantages as mentioned by Behnajady [6]. The sludge is often contaminated and it is classified as chemical sludge with Fe. In addition, this process leads to unwanted side reaction and has poor rate of reaction. Hence, new idea of heterogeneous Fenton process has been developed with the use of iron-free Fenton-like solid catalyst to replace Fe2+/ Fe3+ [7]. This heterogeneous Fenton reaction also takes place in the presence of hydrogen peroxide. However, due to lack of surface area and thicker pore wall, this single-chemical oxidation process still requires highly porous support materials to increase the catalytic activity [8]. Mesoporous materials that have pores diameters in the range of 2-50 nm are potential support material based on their unique properties such as controllable pore size and pore volume, high surface area, high ordered mesostructure, and high thermal stability to act as support materials in Fenton-like system [9-12].
Mesoporous silica in the form of two-dimensional hexagonal structure is formed from the arrangement of silica and the uniform mesopores with the presence of triblock copolymer PluronicP123, (EO)20(PO)70(EO)20 as the structure-directing template [13]. The template is later removed by calcination process to create a material porous structure. Silica is frequently used in formation of mesoporous silica as it is inexpensive, chemically inert and safe to handle [14]. Hexagonal mesoporous silica, or also known as Santa Barbara Amorphous, have been reported by Babaeiab et al. [15] to experience significant damage structure when exposed to high temperature. The secondary pore to its pores walls could disappear from the structure at high temperature and thinner wall of hexagonal mesoporous silica will be produced. Therefore, some modifications have been made by depositary metal iron in the framework of the catalyst [16,17]. This approach could potentially contribute to better efficiency in Fentonlike system. The addition of metal irons such as Fe has been reported to shows positive effect on the internal structure of HMS catalyst [18]. Since then, a lot research works have been carried out and the Fentonlike process employed to be the most attractive method to degrade dye pollutants with the use of supported catalyst [19].
In this study the efficiency of Fenton-like process towards the degradation of organic pollutants has been investigated under variety operating parameters such as the Fe-HMS dosage, initial pH of the solution, amount of H2O2 used concentration of dye, reaction temperature and the addition of salt. The extra of dye removal has been used to determine optimal conditions for the synthesis of the catalyst with regards to the acid concentration, Fe dosage and calcination temperature. The reaction kinetic with this type of catalyst has been investigated. Moreover, the catalyst stability and reusability have also been assessed to demonstrate its potential for application in wastewater treatment.
Materials
Iron nitrate Fe(NO)3.9H2O (99%), Tetraethyl orthosilicate (TEOS) (99%) and hydrochloric acid, HCl (37%) were purchased from the Merck for catalyst preparation. A triblock copolymer, PluronicP123, (EO)20(PO)70(EO)20 (99.99%) from Sigma Aldrich was used as the structure-directing template. Reactive Green-19 (RG-19) (C40H23Cl2N15O19S6Na6) dye was supplied from Merck Company with purity of more than 98% was dissolved in distilled water to be used as the pollutant.
Synthesis of Fe-HMS catalysts
The Fe-HMS catalysts were prepared with various dosages of iron nitrate and acid concentration [16,17]. Iron nitrate (Fe(NO)3.9H2O) and tetraethyl orthosilicate (TEOS) were used as the iron precursor and silicon, respectively. Meanwhile, the non-ionic amphiphilic triblock copolymer PluronicP123, (EO)20(PO)70(EO)20 acted as the structuredirecting template and the hydrochloric acid solution was used as the acidic medium for the synthesis.
In a typical in-situ synthesis, 4 g of surfactant were dissolved in 31 ml of water and then mixture was stirred for 2 hours. 70 mL of different HCl concentration was added into the mixture and was stirred for another 2 hours at room temperature. Then, after the dissolution of block copolymer, the additional of iron nitrate, Fe(NO)3.9H2O was added and 11 mL of tetraethyl orthosilicate (TEOS) was added drop wise to the solution and left under stirring vigorously for 20 hours at 40°C. Then, the resulting solution was transferred into an autoclave for the aging process for one day at 100°C, under static condition. The solid product was then collected in the next filtration step. The precipitate was then left dry in an oven for 24 hours at 80°C. Finally, the surfactant was removed by using calcination at 550°C for 6 hours.
Characterization of the synthesized catalysts
The catalysts were characterized using various techniques to study their respective physical and chemical characteristics. Scanning Electron Microscope (SEM) was used to collect the Fe-HMS surface images. The accelerating voltage and detector current used were at 15 kV and 10 mA, respectively. Energy Dispersive X-ray (EDX) spectrometer was also used in conjunction with SEM to determine the elemental composition of specific spots on the surface of the catalyst. OXFORD INCA 400 EDX system with operating voltages in the range of 0.1 kV to 30 kV was used, with Mn Kα as the energy source. By using a Micrometrics, ASAP 2020 prepared at 77 K, nitrogen adsorptiondesorption isotherms were obtained. The specific surface area and pore size distribution were calculated by using the Brunauer-Emmett-Teller Method (BET) and Barrett-Joyner-Halenda Method (BJH) respectively; in which adsorption branch of the isotherms is in the BJH method. The pore volume was then determined from the plotted graph of nitrogenadsorbed volume against relative pressure [20,21].
Catalytic activity of Fe-HMS catalysts
The degradation of the RG19 dye as the model organic pollutant in this study was carried out based on the reaction the Fenton-like reaction involving hydrogen peroxide, (H2O2) as the oxidizing agent. The preparation of the catalytic synthesis was as desirable in previous works [22,23], with some modifications in terms of the reaction temperature, initial pH of RG-19 solution, Fe-HMS catalyst loading, amount of H2O2 and initial concentration of dye.
100 ml of different concentrations of Reactive Green-19 dye solution was prepared in a series of 1000 ml conical flasks by diluting the dye with 50 mL of deionized water. Next, the pH of the solution was adjusted to the desired values using 0.05 M of HCL solution in order to study the effect of the degradation rate. Different amounts of Fe-HMS catalyst and H2O2 solution were added simultaneously into the conical flask. The prepared conical flasks were placed in a water bath shaker at different reaction temperatures. Lastly, 5 mL of the samples were collected from the reaction vessels every 15 minutes, for up to 120 minutes for analysis.
The color removal was measured by using an UV-Vis spectrophotometer. The concentration of dye solution was taken at different reaction times. The degradation efficiency is calculated as in the following equation, where C0 (mgL-1) is the initial concentration and Ct (mgL-1) is the dye concentration at time, t (min):
Degradation efficiency =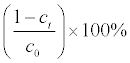 (3.1)
(3.1)
The mineralization percentage efficiency (%) is measured based on Chemical Oxygen Demand (COD). Atomic absorption spectrometer was used to measure the amount of Fe remained after going through degradation step. The standard iron ion solution was first prepared in 0.10 mgL-1 of HNO3 solution to convert a calibration curve. The treated water was first filtered to remove the catalyst from the solution once the decolorization step was completed. Next, the sample was analyzed for the concentration of Fe ion.
Catalyst stability
Once the degradation process was completed, the dye solution was filtered and the solid product was collected to undergo the drying process in an oven 80°C. The dye degradation process was repeated by using this catalyst in order to determine the catalyst stability and reusability.
Kinetic studies of the Fenton-like system
The experimental data were prepared by using 6 conical flasks containing mixtures undergoing different treatment. The kinetics equations derived from the first-order reaction and second-order reaction is used to plot the experimental data against the time taken in minutes. The regression analysis based on this reaction kinetics graph is then performed.
Catalyst characterization
Surface morphology analysis: The surface morphology of prepared catalysts was studied using a Scanning Electron Microscope (SEM). The SEM images show a huge difference in the morphological structure (Figure 1a). The morphology of synthesized catalyst without of HCl was rod-like and it changed to curved rope-like when 2 mgL- 1 HCl was used in the synthesized of the Fe-HMS catalyst. This was because of the presence of acid could result in agglomerated material on the surface as shown in Figure 1a and 1b. This result is considerable with the surface catalysis resulted that will be discussed later.
Energy Dispersive X-ray (EDX) analysis also was performed in continues with SEM to determine the chemical composition on the surface of the prepared catalysts. Table 1 presents atomic percentages of iron, silica, oxygen and carbon of the two catalysts i.e., Fe-HMS-0 mgL-1 HCl and Fe-HMS-2 mgL-1 HCl. Fe elements contributed the lowest composition for both catalyst, followed up by Si and C elements, and the highest composition is O elements.
| Elements | Concentration (wt. %) | |
|---|---|---|
| Fe-HMS-0 mgL-1 HCl | Fe-HMS-2 mgL-1 HCl | |
| Fe | 0.17 | 1.29 |
| Si | 7.90 | 19.45 |
| O | 47.89 | 68.48 |
| C | 44.05 | 10.79 |
Table 1: Chemical composition of the prepared catalysts.
The amount of Fe ions bonded on the surface of the prepared catalysts was found to increase with the presence of acid in the synthesize mixture. This is due to the fact that acid might block Fe ions from breaking into the structure of silica. On the other hand, the actual Fe concentrations in both samples are slightly different, in which higher Fe ions was detected in the catalyst without acid (3.468 mgL-1) than catalyst with acid (0.741 mgL-1).
Characteristics of Fe-doped catalysts with Fe dosage varied to two different levels were investigated by using surface morphology analysis to investigate Fe affect the morphology of the catalyst. Similarity, variation in the Fe dosage could leads to huge difference in the pore structure. As seen in Figure 2b, a curved rope-like material is clearly seen at lower Fe loading (3 wt. Fe). The change of Fe loading from the previous preparation of catalyst (change in acid concentration) has similar texture with the original catalyst without acid. It may be indicates the presence of similar microstructure. However, with higher loading of Fe, the morphology of Fe-HMS changes to rod-like material as shown in Figure 2b.
Surface analysis: Structural characteristics of Fe-HMS catalyst without and with the presence of acid were studied based on N2 adsorption-desorption at 77 K. Based on the results shown in Table 2 and Figure 2a and 2b, the presence of acid has significantly influenced the physical properties of both samples. It could be detected that an adsorbate monolayer was formed for both catalysts, followed by the multilayer formation at relative high pressure of 0.5 to 0.8 for the sample without acid and within range between 0.6 and 0.8 P/P0 for the other sample. The increase in the P/P0 that there was a decrease in pore size. This can be seen in the BET surface area in which the catalyst without acid has to form multilayer smaller pore size than suggested catalyst the one with acid. Both samples show typical type-IV isotherm in Figure 2b, mainly due to the capillary condensation and desorption of nitrogen. According to IUPAC classification [19-26], this type of hysteresis with loop and the shift of sharp inflection of P/P0 are typical for 2D-hexagonal mesoporous material.
| Catalyst | BET Surface Area (m2 g-1) | BJH Pore Size (nm) | Pore Volume (cm3 g-1) | ||
|---|---|---|---|---|---|
| Micropore Volume | Mesopores Volume | Total Pore Volume | |||
| Fe-HMS-0 mgL-1 HCl | 646 | 5 | 0.023 | 0.746 | 0.769 |
| Fe-HMS-2 mgL-1 HCl | 601 | 6 | 0.056 | 0.722 | 0.779 |
Table 2: Surface analysis of the prepared catalyst.
The pore size distributions in Figure 2b indicate that both mesoporous catalysts had narrow and rather wide distribution. Additionally, a wider distribution can be noticed in the case of the catalyst pore size to indicate that the volume of mesoporous was higher compared to the catalyst with acid with a difference of 0.023 nm. The total pore volume indeed was found to increase when acid was used in the synthesis as supported by result in Figure 2b and Table 2. Both samples show maximum symmetric peaks at about 6 nm, but the intensity of Fe-HMS-2 mgL-1 HCl peak is higher compared to that of Fe-HMS-0 mgL-1 HCl, which reveals clearly suggests larger pore volume.
It should be noted that the BET surface area of Fe-HMS-0 mgL-1 HCl catalyst was slightly higher (646 nm) than that of Fe-HMS-2 mgL-1 HCl catalyst (601 nm). The rod-like material as seen in the SEM images in Figure 1a by the catalyst without acid suggested that its surface area was larger compared to the other catalyst (curved-rope material). This is might due to thinner wall that resulted with the use of acid in the synthesis of Fe-HMS framework. The total pore volume was inversely proportional to the surface area as shown in Table 3. The total pore volume of Fe-HMS-0 mgL-1 HCl was smaller compared to that of Fe- HMS-2 mgL-1 HCl, with a difference of 0.01 cm3 g-1 only. On the other hand, the range of pore size for the mesoporous material is within 2 up to 50 nm, and thus, the BJH pore sizes shown in Table 3 are acceptable. Both catalysts had pore sizes above 2 nm or more accurately; 5 nm for the Fe-HMS-0 mgL-1 HCl and 6 nm for the Fe-HMS-2 mgL-1 HCl.
| ∆G˚(kJ mol-1 ) | Ea (kJ mol-1) | ∆H˚(kJmol-1) | ∆S˚(J mol-1K -1) | |
|---|---|---|---|---|
| Temp 25 | 8.93372 | 37.0147 | 34.4831 | -0.1588 |
| Temp 35 | 8.09186 | |||
| Temp 40 | 7.49284 |
Table 3: Thermodynamic parameters for the catalytic degradation of RG19 through the Fenton-like reaction.
Performance in catalytic degradation of RG-19
The first experimental were carried out without heterogeneous catalyst (blank sample), but with the presence of hydrogen peroxide only. Figure 3a shows reaction between dye molecules and hydroxyl radical as Reactive Green-19 dyes under heterogenous14% decolorization within 120 minutes. This clearly suggests the role of catalyst itself to increase the rate of reaction. The decolorization of the dye done to absorption process was studied for each experiment in which the process was allowed to occur the first 15 minutes (the figure shows -15 minutes). For each experimental gave very low degree of decolorization (lower than 1%) to indicate poor adsorption of the dye by the catalysts. As such, decolorization should be mainly attributed to the Futon-like reaction.
Results in Figure 3a suggest that concentration of hydrochloric acid positively affected on the degradation of Reactive Green-19 dye. Higher concentration of hydrochloric acid increscent improved color removal. Catalyst with no acid clearly had slower reaction compared to others with only 34.24% of decolorization within 120 minutes of reaction. The higher amounts of hydrochloric acid used in synthesis of Fe-doped HMS catalyst resulted in the highest catalytic activity with 95% color removal in 120 minutes. In the catalyst synthesis, hydrochloric acid is needed in the hydrolysis step of teraethoxysilane (TEOS) and it should be suitable for the assembly of triblock polymer P123, as there is a combination of electrostatic and hydrogen-bonding interactions.
However, improvement in the decolorization by catalysts with between 1.5 mgL-1 and 2 mgL-1 was rather small. As such, further increase in the amount of HCL used beyond 2 mgL-1 should not be attempted.
Effect of Fe loading in Fe-doped HMS as catalytic activity
The positive effect increasing dosage of Fe in the catalyst as seen in Figure 3b suggests the complementary roles of Fe with HMS catalyst. Active sites are needed for the acid while HMS played the role as a good support material reaction. Erdem et al. [24] outlined that metal ions itself are involved in self-assembly process as protons. Therefore, the reducing amount of Fe might affect the hydrogen bond in formation of meso-structured, as highlighted in the previous section. In addition, catalytic performance of modified Fe-HMS catalyst was better with the presence of higher loading of Fe (Figure 3b). Within 60 minutes of reaction, most of the catalysts (with Fe loading higher than 9-wt%Fe) achieved approximately 90% of decolorization, while Fe-HMS-7wt%Fe achieved 85% decolorization and Fe-HMS 3wt%Fe could only managed to achieve 53.59% decolorization.
The increase in the Fe loading from 7wt% to 13 wt% resulted in only a little improvement in catalyst activity. However, considerate potential loading of Fe in the process, the highest Fe ion (Fe-HMS- 13wt%Fe) was taken as the loading to be further investigated in the following section.
Effect of operating conditions on catalytic activity
To find the optimal parameters for best efficiency of Fenton-like oxidation, effects of reaction temperature, initial pH of dye, Fe-HMS loading, hydrogen peroxide dosage, and initial concentration of dye and addition of salt were studied.
Effect of reaction temperature: The effect of temperature on effectiveness of Fenton-like oxidation was investigated in the 25°C - 40°C range and the other parameters were fixed (pH 3, 100 μL H2O2, 100 mgL-1 of dye, and 1 mgL-1 of Fe-HMS catalyst). In the first -15 the adsorption of Reactive Green-19 dye was formed to be minimum with decolorizations below 1%. Thus, Fe-HMS catalyst was conducted to play an important role in removal of the dye from the solution.
The run conducted at 25°C showed relatively lower removal. The highest decolorization was achieved 96.4% within 120 minutes. For the runs at 35°C and 40°C, decolorizations of nearly 99% were achieved in the same direction. Previous researchers report that the optimal temperature for the process was below 45°C [25]. The generation rate of hydrogen peroxide from the high temperature could accelerate the effectiveness of the Fenton-like oxidation. The increase in the temperature also provided more energy for the dye molecules to overcome the interaction between them. Therefore, positive effect on treatment efficiency can be clearly seen in Figure 3c as the decolorization increased with the increase in the temperature. It was proven that at high temperature (40°C), shorter period of time to reach approximately 98% degradation within just 60 minutes of reaction.
As presented in Table 3, the thermodynamic parameters of the catalytic decolorization of Reactive Green 19 dye are summarized for both Eyring and Arrhenius equations. The positive value of Gibbs free energy, ΔG° indicates that the oxidative reaction using the Fentonlike reagent is non-spontaneous in nature. The activation energy (Ea) was calculated from the Arrhenius plot is low (37.0 kJ mol-1) with the presence of Fe-HMS catalyst. Olajire et al. [1] reported that metal activation significantly reduce the activation energy in compared to the run in the absence of a catalyst.
The Fenton oxidation is an endothermic process and results in Table 3 are consistent with reported area [1,26]. The positive value of enthalpy, (ΔH°) suggests that the process is an endothermic one. There increase of the decolorization with the increase in the reaction temperature also correctly suggest that it is an endothermic process (Figure 3c). Therefore, the results in Table 3 are valid theoretically and experimentally. Next, there was a decrease in the degree of of randomness as the value of entropy (ΔS°) is negative [27].
Effect of initial pH of reactive green-19: pH plays an important role as industrial effluent could have variable pH value that may affect the reaction mechanism, especially in production of hydroxyl radicals. A series of blank run (without catalyst) was first conducted within the first 15 minutes (-15 minutes on the graph) to prove the effectiveness of the Fenton-like reagent. A sluggish activity compared to the next 120 minutes suggested that the reaction was way better with the presence of Fe ions.
Figure 3d shows that the decolorization efficiency was high in strong acidic conditions (pH 2~ pH 3) of compared in weak acidic conditions (pH 4~ pH 6). At initial pH value of 2.00 and 3.00, the decolorization efficiencies were above 96% within 60 minutes as compared to the slower reaction rate in weak acidic conditions. Higher pH leads to the decreasing oxidation potential of hydroxyl radicals and while causing Fe3+ to undergo precipitate reduced the concentration of Fe3+ in the dissolved state. This could be observed in the figure. Within 120 minutes the run at pH 6 reached only 7.5% decolorization efficiency while at pH 5 reached 79.2%.
Nonetheless, there is a slightly difference between decolorization efficiency at pH 2 and pH 3. pH 3 clearly showed fastest rate of reaction to remove the color almost completely. Thus, it can be concluded that too acidic solution would reduce the effectiveness of the Fenton-like process. As a result, the optimal pH was obtained at pH 3. Several literatures [28,29] also reported similar results with other model components. They also achieved above 95% color removal at pH 3. In addition, [1,27] found that the increase in the pH above 1.45 would decrease degradation efficiency in the case of Methylene Blue dye.
Effect of Fe-HMS catalyst loading: Effect of Fe-HMS catalyst loading on the Fenton-like oxidation were also investigated with variable catalyst loading (0.5, 1.5, 3.0, 5.0 and 1 mgL-1 catalyst loading) while the other parameters were fixed in 40°C, pH 3, 100 μL of H2O2, and 100 mgL-1 of Reactive Green-19 dye. Based on Figure 3e, the absence of solid (Fe-HMS) catalyst caused poor rates of reaction with the highest degradation percentage is about 2.98% at catalyst loading of 5.0 while 0.5 mgL-1 Fe catalyst only achieve 0.12% of decolorization.
From previous section, only run at pH 2 and pH 3 achieved more than 80% of decolorazation in 30 minutes. In this study, 0.5 mgL-1 of Fe catalyst loading reacted almost 90% in 30 minutes. In 90 minutes, almost complete decolorization was achieved. Additionally, it can be seen that at 90 minutes, 3 mgL-1 Fe loading reached its maximum decolorazation first, followed by 5 mgL-1 Fe. The decolorization by 5 mgL-1 Fe only increased very slightly from 60 minutes to 90 minutes of compared to that with 3 mgL-1 Fe. Results in Figure 3e suggest that even with a small amount of iron loading (0.5 mgL-1 Fe-HMS catalyst), the decolorazation obtained within 30 minutes was nearly 90%.
Based on Ertugay and Acar [9], the addition of Fe3+ ions to the reaction system should not exceed 3 mgL-1. At higher Fe loading, brownish particles appeared and it could block the adsorption needed for the Fenton-like process. This contradicts with experimental results in this study as the decolorization continued to increase with increase in the Fe catalyst loading [9,29].
In a nutshell, higher amount of Fe loading adding up to the reaction will accelerate the reaction with hydrogen peroxide to produce more hydroxyl radicals. The optimal condition was achieved at 3 mgL-1 of Fe catalyst loading where the decolorization efficiency reached almost 99%, within 90 minutes.
Effect of hydrogen peroxide dosage: Based on the following reaction, the number of hydroxyl radicals should increases with the increase in the of hydrogen peroxide dosage.
H2O2+ HO• → H2O+ HO2•
In order to prove this relationship a series of experiments were performed by varying the dosage of hydrogen peroxide (25, 50, 150, 200, and 300 μL). The initial pH was kept at 3 with 3 mgL-1 of Fe loading, and the reaction temperature was set at 40°C. In the first -15 minutes, all runs had almost the similar performance, approximately above 1.4% of decolorization. The presence of a Fe-HMS catalyst as clearly increased the treatment efficiency.
The data obtained from this section are slightly different from those in earlier section. Results in Figure 3f indicate that the increase in the hydrogen peroxide dosage improved the decolorization efficiency, which concludes that the hydroxyl radicals did affect the process. Results in the first 15 minutes suggest that hydrogen peroxide dosages above of 150 μL had similar efficiency, with 86, 81 and 75% of decolorization efficiency, followed up by 50 μL of H2O2 with 54% and the slowest reaction was shown by the run with 25 μL of H2O2 (21%). Within 60 minutes, 50 μL of H2O2 caused the highest reaction (99.9%). The increase in the H2O2 dosage would lessen the efficiency of the reaction, and this might be due to the scavenger effect on the hydroxyl radicals. Besides, hydrogen peroxide dosages exactly 50 μL could have reached their equilibrium within 45 minutes. However, from the literature Ertugay and Acar [9] mentioned that the addition of more than 125 μL of H2O2 would reduce the color removal by about 34%. This might due to the difficulties of several structures of dye molecules to be attacked by hydroxyl radicals. In this study, 50 μL of H2O2 was chosen as the optimal for this parameter to be used in the subsequent stage [9].
Effect of initial concentration of dye: The first -15 minutes on Figure 3g indicate slow progression of reaction for 4 samples with different initial dye concentrations. The highest dye concentration (120 mgL-1) resulted in an increase only about 1.12% hydroxyl radicals alone are not strong enough to attack dye molecules. This is in contrast with other samples as decolorazation shown are above 10 within these -15 minutes. Lower initial concentration of dye tends to result in faster decolorization even though with the absence of iron ions.
A series of experiments were then conducted at pH 3 with variable of concentration from 20 to 120 mgL-1 to study the effect of the model pollutant concentration. For, 120 mgL-1 the decolorization process rather slowly with 95.3% decolorization within 60 minutes. Meanwhile, the lowest concentration (20 mgL-1) resulted in almost 100% within the same time. Initial concentration of dye seemed to affect the Fentonlike oxidation reaction due to the percent of different amounts of dye molecules in the system. Since the dosage of Fe and hydrogen peroxide were fixed, the numbers of hydroxyl radicals remain the same. Thus, the same amounts of hydroxyl radicals would be used to attack higher number of dye molecules in the solution of 120 mgL-1 dye.
Higher initial concentration of Reactive Green-19 could reduce the decolorization efficiency by approximately 1% for each initial concentration of dye and the obtained results were in good agreement with that reported by Zuorro and Lavecchia [30]. However, different types of dye have their own initial concentration and the best results for the rate of decolorization of Reactive Red 19 and Reactive Blue 19 were obtained with 50 mgL-1 for dye concentration while it was 4 mgL-1 for Amaranth Red Dye [9,19,30]. The lower number of dye molecules initially seemed to result in higher decolorization rate.
Effect of addition of salt: Salt also have been considers to effect wastewater treatment and one of the steps in dyeing industry involve the used a large amount of salt. In this study, the effect of addition of salt on decolorization of dye was assessed with 0, 1.2, 3.5, 7.0 and 11.7 mgL-1 of sodium chloride. The presence of NaCl at 1.2 mgL-1 seemed to reduce the decolorization from 96% (without NaCl) to only 42% within 60 minutes, to indicate significantly slower a slow motion of Fenton-like process. Other sample with 3.5 mgL-1 of NaCl led to 35.5% decolorization within 60 minutes, followed by 7.0 mgL-1 (32.6%) and 11.77 mgL-1 (30.1%). The higher amount of salt used, the lower the decolorization rate. On the other hand, higher amount of salt did not significantly affect the absorption process in the first 15 minutes. In view of this, it is proven that production of anion (Cl-) has negative effect on the Fenton efficiency. Chloride ion has lower oxidation potential (1.36 V) than that of hydroxyl radical (2.80 V) it could lead to oxidation of chloride ion by hydroxyl radical. Thus, the amount that remained for the decolorization of dye could be reduced (Figure 3h).
The negative impact on Fenton-like oxidation was consistent with the result reported by Emami et al. [11]. Meanwhile, Abou-Gamra reported that Fenton-like reaction was unaffected if the concentration of NaCl was increased up to 0.340 mgL-1. This was slightly different with the result in this study as salt kept on reducing the Fenton-like reaction effectiveness until 11.77 mgL-1. The difference could be due to the difference in the structure of dye. Thus, the large amount of salt used in textile process negatively affected decolorization efficiency of dye molecules.
Mineralization of reactive green-19
By definition, chemical oxygen demand or COD is the amount of oxygen required to oxidize any organic matter in the water sample by means of a strong chemical oxidizing agent (potassium dichromate oxidation was used in this study). The initial COD of Reactive Green-19 obtained from this experiment was 222 mgL-1, even though the initial concentration of dye used was 100 mgL-1.
Results in Figure 4 indicate that the removal of COD increased with the cause of the reaction. However, decolorization efficiency was significantly higher than the COD removal with the percentage difference of 29% within 120 minutes. This could be due to the fact that although Reactive Green-19 dye had undergoes almost complete degradation, it was an incomplete oxidation of Reactive Green-19.
The result above can be verified from ‘absorbance vs. wavelength’ plot of shown in Figure 5. There are two peaks shown by the original sample (dye only) on Figure 5. The highest peak presents the wavelength for Reactive Green-19 dye, while second peak suggest complete mineralization did not take place. Subsequently, the initial of the second peak decreased with reaction time, when the mineralization process started to occur with the addition of the Fenton-like’s reagent.
However, Zuorro and Lavecchia [30] mentioned that the COD removal for Reactive Green-19 dye only achieved above 70% after several hours. Even in some case, the mineralization percentage only approached 80% after 10 hours. Thus, COD removal could indicate the percent of mineralization that it depends on molecular structure of the organics themselves [30].
Kinetic study for heterogeneous Fenton system
Figure 6 shows the catalytic decolorization of Reactive Green-19 dye by using different types of the Fenton-like treatment. In the heterogeneous Fenton process (Fe-HMS catalyst and H2O2); faster decolorization was achieved, which also has higher value of constants for both first and second order models (Table 4). Besides, heterogeneous Fenton process itself has the combination process of adsorption and oxidation, which relied on availability of high surface area.
In this study, there were still slow reactions occurred with the presence of either Fe or H2O2 only, where the efficiency of Fenton-like oxidation decreased from 98% until 12% and 28% within 120 minutes. As discussed in earlier section (effect of addition of salt), the presence of chloride ions could reduce the effectiveness of Fenton-like oxidation. The difference of removal percentage with and without the presence of salt in the reaction is approximately 55%, within 120 minutes (Figure 6).
The pseudo first and second-order plots are made using the equations. The linear trend line of (ln Co/Ct) and (1/Ct-1/Co) against reaction time in Figure 7a and Figure 7b gives the values of correlation coefficients, (R2) and rate constant (k) as summarized in Table 4. The fastest decolorization, which involved the use of heterogeneous Fenton catalysts have higher values of R2 (>0.9399) and approaching 0.9599 for the pseudo first order model as compared to relatively poorer fittings to the pseudo second order model. The lowest R2 is shown by the reaction involving the use of hydrogen peroxide and salt, in which the value are 0.5831 for the first order model and 0.6112 for second order model.
| 1st Order | 2nd Order | |||
|---|---|---|---|---|
| Types of treatment | R2 | k1 (min-1) | R2 | k2 (L mg-1 min-1) |
| H2O2 only | 0.6699 | 0.0033 | 0.7219 | 4.0E-0.5 |
| Fe-HMS only | 0.7816 | 0.0012 | 0.8014 | 1.0E-05 |
| Fe-HMS+H2O2 | 0.9399 | 0.0379 | 0.8045 | 0.0053 |
| H2O2+NaCl | 0.5831 | 0.0019 | 0.6112 | 2.0E-05 |
| Fe-HMS+NaCl | 0.8374 | 0.0017 | 0.8316 | 2.0E-05 |
| Fe-HMS+H2O2+NaCl | 0.5889 | 0.0052 | 0.7309 | 7.0E-05 |
Table 4: Kinetic parameters for the reaction at various conditions.
The reaction by involving Fe-HMS catalyst, H2O2 and salt seems to favour the first order model on the basis of higher value of R2. This result is in a good agreement with the study by Olajire et al. reported higher correlation with the second-order reaction [1,29].
Reusability and stability of the catalyst studies
The reusability of Fe-HMS catalyst was investigated by repetitive use of the catalyst, as shown in Figure 8. The catalyst was filtered and tested using atomic absorption spectrometer to detect the leaching of iron ions at the end of the process. The amount of Fe was 1.439 mgL-1 Fe.
The reaction rates for the first two cycles were found to decrease due to the deactivation of the catalyst [7,29,30]. Nevertheless, the next two cycles showed the increment of the decolorization efficiency by approaching almost similar activity with the previous run. There was no color change in each cycle, where the original color of the catalyst was brown. The Fe-HMS catalyst could be reused with almost the same catalytic activity.
Condition in the preparation of iron-doped hexagonal mesoporous silica catalyst (Fe-HMS) using direct synthesis method was found to affect their catalyst activity. The presence of acid in the synthesis mixture could increase the reaction for up to a HCL concentration of 2 mgL-1. Meanwhile, the addition of Fe ions above 7 wt%- Fe led to excellent improvement, in the efficiency. Thus, 13 wt% of Fe was selected as optimal level for the development of Fe-HMS catalyst. The characterization results suggested that little change in the synthesis condition of Fe-HMS catalyst, could lead to huge difference in the pore structure. The decolorization of Reactive Green-19 by the Fentonlike reaction was also studied by varying the experimental conditions. The most effective decolorization was achieved pH 3 and reaction temperature of 40°C with near complete decolorization efficiency. By manipulating these parameters, the reaction time to reach complete decolorization was shorted from 120 minutes to 90 minutes. Meanwhile, the other optimal parameters were 3 mgL-1 Fe catalysts loading and the addition of 50 μL of H2O2 solution. On the other hand, a small quantity of salt negatively affected the reaction. The kinetic study led to conditions that pseudo first order model accurately fits the date. The reusability potential of Fe-HMS catalyst was also proved using 4 cycle of catalytic runs with activity drop lower that 20%.
A Research University Grant (814144) from Universiti Sains Malaysia and a PRGS Grant from Ministry of Higher Education (6762001) are gratefully acknowledged.