Research Article - (2017) Volume 5, Issue 8
The experiment was conducted under in vitro and field conditions to observe the effect of bio-agents, botanical and fungicides against Alternaria triticina. Eight treatments were taken up with three replications and data collected was analyzed using CRD. Maximum inhibition percent mycelia growth was observed in Propiconazole (89.72%), Hexaconazole (88.44%), Vitavax (87.70%), followed by Trichoderma harzianum (85.50%), Trichoderma viride (83.30%), Pseudomonas fluorescens (80.73%) and neem leaf extract (73.57%) as compared to control (0). An experiment was conducted under field condition to observe the effect of bio-agents, neem leaf extract and fungicides against Alternaria triticina. Eight treatments were taken up with three replications and data collected was analyzed using RBD. Maximum plant height (cm) was observed in T. viride (78.82 cm) followed by T. harzianum (78.27 cm) as compared to control (70.04 cm). T. viride was significantly superior as compared to other treatments. Minimum disease intensity per cent and production of wheat was recorded in treatment Propiconazole @0.1% (18.24% and 37.00 q/ha respectively) followed by Pseudomonas fluorescens @0.5% (20.51% and 30.44 q/ha), as compared to control (43.18% and 20.41 q/ha). Propiconazole was significantly superior as compared to other treatments.
Keywords: Alternaria blight; Trichoderma spp; Fungicides; Neem leaf extract
Wheat (Triticum aestivum L .) is the world's most extensively grown crop and important staple food. There are several constraints limiting the potential yield of wheat. Among them foliar blight has recently emerged as major concern throughout the world [1]. In India, foliar blight of wheat had been noticed as early as 1924 [2], but it was not of much consequence till recently. In the recent past, with the change in cropping system, foliar blight has now become a major disease far and wide in our country causing 2.72 to 36.24% yield losses under different agro climatic zones. In India, foliar blights of wheat are considered as one complex, which includes leaf blight caused by Alternaria triticina Prasada and Prabhu and spot blotch caused by Bipolaris sorokiniana . In the Indogangetic plains, covering the entire north-western and north-eastern plains, rice-wheat rotation is the dominant cropping sequence. It has been commonly observed that the intensity of foliar blights has considerably increased in the rice-wheat system [3]. During last decade multilocational surveys for determining incidence of foliar blights of wheat have been conducted in eastern U.P, Bihar, Haryana, Punjab, Delhi, Gujrat and Rajasthan [4-15]. In view of the growing concern about foliar blight of wheat, it was considered desirable to study the incidence of the disease and the causal organisms in Allahabad region where wheat (Triticum aestivum L .) is main food crop. Leaf blight caused by Alternaria triticina is the major disease in irrigated wheat in Vidarbha region of Maharashtra. Alternaria leaf blight was first reported from Maharashtra in 1924 [2]. The disease initially appears as small and irregularly scattered chlorotic lesions on the leaves in last week of December. As the disease progresses, several spots coalesce and cover the whole or part of the leaf giving it a blighted' appearance. Heavily infected fields show a burnt appearance [5,16].
The normal sown as well as late sown irrigated wheat varieties were found heavily infected with Alternarialeaf blight during January and February in Vidarbha region, causing considerable losses in the grain yield of irrigated wheat. Therefore, this trial was formulated to estimate the losses caused due to leaf blight disease [6,17].
The application of two irrigations reduced the severity of foliar blight as compared to no irrigation [7] Sreshta reported that low or imbalance soil nutrient levels predispose plants to more severe leaf blight attack found low incidence of disease when wheat crop was sowing on 30th November as compared 20th December [18-22]. Among the different diseases caused by the genus Alternaria blight disease is one of the most dominant one that causes average yield loss in the range of 32-57%. Symptoms of this disease include presence of irregular, often circular brown to dark brown colour leaf spots on the leaves with concentric lines inside the spots. Often the circular spots coalesce to form large patches resulting in the leaf blight. In several cases, small dark coloured spots are also formed on pods and tender twigs [23]. For Alternaria blight management, early sowing of properly stored clean certified seeds after deep ploughing along with clean cultivation, timely weeding and maintenance of optimum plant population, avoidance of irrigation at flowering and pod formation stages are some of the steps to be followed for an efficient management of the disease [24].
Conidiophores of majority of the species of Alternaria produce asexual spores (conidia) measuring between 160-200 μm long. Under in vitro conditions, sporulation occurs at a temperature range of 8-24°C, where mature spores occur after 14-24 h. Optimum temperatures are between 16 and 24°C where sporulation time ranges from 12 to 14 h. Moisture in the presence of rain, dew or high humidity are essential for infection and a minimum of 9-18 h are required for majority of the species [10]. Continuous moisture of 24 h or longer practically guarantees infection [11]. Relative humidity of 91.5% (at 20°C) or higher will result in the production of large numbers of mature spores in 24 h [10].
The present investigation was carried out during 2014-15 Rabi season at the Department of Plant Pathology Central Research field, Sam Higginbottom Institute of Agriculture Technology and Sciences (Deemed to-be University) Allahabad, India. A field study conducted to Present experiment with appropriate statistical design was conducted in the field conditions by adopting Randomized Block design (RBD) with eight treatments and each treatment was replicate three times [12-20]. The details of materials and methods followed during the course of present investigation are described below.
The seeds of variety PBW-343, which is highly susceptible to leaf blight were collected from Sam Higginbottom Institute of Agriculture Technology and Sciences (Deemed to-be University) Allahabad, (SHIATS), plant extracts viz. extracts of leaves of neem (Azadirachta indica and three BAU-Biofungicide (Trichoderma viride , Trichoderma harzianum , Pseudomonas fluorescens based preparation and which obtained from the Disease Resistance Laboratory) were used. Propiconazole Hexaconazole, Vitavax was used as chemical check.
Preparation of plant extract
Plant material are chopped in required quantity of water (1:1 w/v), boiled for 30 min and then strain through cheese cloth to obtained standard plant extract solution (100%) [21-23]. The extract is centrifuge at 5000 rpm for 5 min and the clear supernatant was used as stock solution.
Application of neem leaf extract (Azadirachta indica)
Neem leaf extract was prepared according to Paul and Sharma. 400 g (fresh wt) mature leaves were homogenized in a pre-chilled pestle and mortar using chilled, sterilized distilled water. The extract was filtered through four layers of moisture muslin cloth. The final volume was adjusted to 1000 ml with distilled water. The filter ate was centrifuged at 2000 g, 4°C for 15 min. The supernatant thus obtained was designated as concentrated leaf extract. Dilution of 1:2 was made from this concentrated extract [25].
Application of Trichoderma viride, Trichoderma harzianum and Pseudomonas fluorescens
Talcum based formulation of Trichoderma viride manufactured by Yash Biotech Pvt. Ltd; Allahabad was used for field experiment. Before applying the talcum based formulation of T. viride in the field the c.f.u was checked in the laboratory seed treatment @10 g/kg of T. viride was used. Foliar spray of T. viride was at 50 DAS @10 g/l of water and the subsequent spray was given at 15 days interval as suggested by Rathi and Singh [26].
Preparation of fungicidal spray solution
The spray solution of a desired concentration was prepared by adopting the following formula.
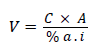
Where,
V=Volume/weight of commercial fungicide ml or g
C=Concentration required
A=Volume of solution to be prepared
% a.i.=percentage of active ingredient in commercial product.
c.f.u count of T. viride and T. harzianum formulation
One gram of Trichoderma powder was weighed and the volume was made up to 10 ml with sterilized distilled water and was shaking well (1:10) inside laminar flow hood. Out of this suspension 1 ml was taken out and transferred to 9 ml of sterilized distilled water in a test tube (1:100). Serial dilution were made similarly by transferring 1 ml of each suspension to the subsequent tubes to get 10-7 dilution. 1 ml of 10-7 suspension was transferred to sterilized Petri plates. 15 ml of melted and cooled PDA medium was poured in these plates. The plates are rotated gently to give a uniform distribution and allowed to solidify [27-32]. The plates were incubated in an inverted position at 25 ± 2°C. After 3 days, average numbers of colonies were calculated per plates of T. viride and T. harzianum . Four colonies each were found per plate and the number of colony forming unit (c.f.u) present in 1 g was calculated by the following.
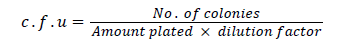
c.f.u count of Pseudomonas fluorescens formulation
Colony forming unit (c.f.u) of Pseudomonas fluorescens was done to check the viability and spore load in formulation before their application. One gram powder formation of P. fluorescens was weighed and added in 9 ml of sterile distilled water, shaken well and label as 10-1 (1:10). Made dilution serially up to 10-8 as given below: From the first dilution transferred 1 ml of suspension to the dilution blank 10-2 with sterile pipette diluting the original suspension to 100 times (1/100) or 10-2). From the 102, transferred 1 ml of suspension to 10-3 dilution blank with sterile pipette, thus diluting the original sample has been diluted 100000000 (10-8) [33-37]. From the dilution 10-4-10-8 transferred 1 ml of suspension while in motion, with the respective pipettes, to sterile petri dishes (Table 1). Added 15 ml of King’s B melted cold medium, mixed the contents of each plate by rotating gently. Allowed the plates to solidify and incubated in inverted position for 24 to 48 hrs at 25 ± 2°C [34].
| S. No. | Per cent leaf area covered |
Grade (x) | No. of leaf falling in grade (y) |
Disease rating (x*y) |
|---|---|---|---|---|
| 1 | 0 | 0 | - | - |
| 2 | 1 | 1 | - | - |
| 3 | 02-Oct | 3 | - | - |
| 4 | Nov-25 | 5 | - | - |
| 5 | 26-50 | 7 | - | - |
| 6 | 50> | 9 | - | - |
| Total | - | - | ||
Table 1: Scale of disease assessment.

Disease intensity (%) was calculated by using the following formula
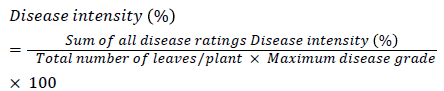
in vitro experiment
The in vitro trial was laid out in completely randomized design (CRD) with three replications and eight treatments including check in the experimental laboratory of department of Plant Pathology. The management of leaf blight of wheat, with bio-agents, neem leaf extract and fungicides was tested applying poison food and dual culture techniques against Alternaria triticina. The observation of the mycelial growth inhibition per cent was recorded at 1 to 7 days [38-45].
Cleaning and sterilization of glassware
The glasswares (Petri dishes, pipettes, conical flasks, test tubes etc.,) used in the experiment were thoroughly washed with detergent powder and air dried. The Petri dishes and pipettes were wrapped in clean paper and sterilized in hot air oven at 160°C for 2 hrs.
Poisoned food technique
The poisoned food technique was followed to evaluate the efficacy of propiconazole (Tilt 25 EC) @0.1%, hexaconazole @0.5% and vitavax @0.25% in laboratory against Alternaria triticina with three replications each. Mycelial disc of 5 mm size from seven days old cultures was cut out by a sterilized cork borer and one such disc was placed at the center of each agar plate. Check treatment was maintained without adding any fungicides to the medium [46,47]. After incubation for 24 to 168 hrs, at room temperature, radial growth was measured till fungus attained maximum growth in check plates. The efficacy of the fungicides was expressed as percent inhibition of mycelial growth over check, which was calculated by using the formula by Vincent [14,48].
Preparation of Potato Dextrose Agar (PDA) medium
The culture media used in experiment were prepared according to the standard formula given by for isolating and growing of pathogen Alternaria sp. Potato dextrose agar (PDA) medium was used the composition of PDA is as follows composition of PDA [49]. Two hundred gram washed, peeled and sliced potatoes were boiled in 500 ml of distilled water in a sauce pan till they were easily penetrated by glass rod. The extract obtained was filtered through muslin cloth and all the liquid was squeezed in a beaker, 20 g agar was added bit to the rest of 500 ml hot water to dissolve. Then 20 g of dextrose was added [50]. Volume of broth was made up to 1000 ml by adding more distilled water. Then 200 ml of this solution was dispensed in conical flasks [51-53]. These conical flasks were plugged with non-absorbent cotton and sterilized at 121°C at 15 lbs/inch2 for 20 minutes in an autoclave.
Preparation of nutrient agar medium
Boil one liter of distilled water, to this add beef extract, when this was dissolved added peptone and NaCl. The pan was then taken down from the flame and agar was added with regular stirring. pH (7.0-7.2) was tested with the help of pH paper [54]. The media was transferred to test tubes and conical flasks and plugged with cotton. The media was then subjected to autoclave at 15 lbs pressure per sq inch or 121°C for 20 minutes [55].
Colony growth inhibition assay with Trichoderma spp. and Pseudomonas fluorescens in dual culture method
Firstly, the antagonistic activity of Trichoderma viride , T. harzianum and Pseudomonas fluorescens against Alternaria triticina was studied in dual culture method [15]. So the antagonist was evaluated by dual culture technique. The pathogen was inoculated on one side of the petri plate filled with 20 ml of PDA and antagonist was inoculated at exactly opposite side of the same plate by leaving 3-4 cm gap. For this, actively growing 7 days old culture was used. Dual inoculation of the pathogen and an antagonist was set up [56-60]. Culture discs of 5 mm dia were cut from the periphery of the actively growing colonies using a sterilized cork borer. Disc of test fungus was placed aseptically at the edge of the Petri plate. These plates were incubated at 25 ± 1°C for 48 hours. Mycelia disc (5 mm) of antagonist was inoculated on opposite side of Petri plate three days after the pathogen to adjust for the slow growth rate of the pathogens. Paired cultures were again incubated at 25 ± 1°C for 24-168 hours and observed periodically. Then antagonistic fungi were tested against Alterneria triticina . Each set was made in 3 replicates [61,62].
T1=Alterneria triticina +Trichoderma harzianum
T2=Alterneria triticina +Trichoderma viride
T3=Alterneria triticina +Pseudomonas fluorescens
Mycelium Inhibition % calculated by the following formula
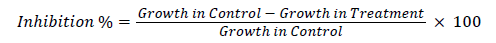
Isolation of Alternaria triticina
Wheat crop sowing characteristic symptoms of leaf blight were collected from Center Research Farm SHIATS, Allahabad. Infected leaf blight samples were thoroughly washed in running tap water and cut into small pieces of 2-5 mm size having half healthy tissues. After surface sterilization with Sodium hypochlorite (0.1%) for 30-60 seconds, infected leaf blight pieces were thoroughly washed three times in sterilized water and blotted dry on clean sterile blotting paper to remove the moisture. These pieces were transferred aseptically into Petri plates containing potato dextrose agar (PDA) medium [63]. The inoculated Petri plates were incubated in a B.O.D incubator at 28 ± 1°C. After 48 hours of incubation, mycelia growth developed at the margin was transferred to PDA slants. The pathogen culture was maintained on PDA in culture tubes and stored in refrigerator for further use.
Morphological studies of the pathogen
A morphological study of the pathogen was conducted from pure culture of Alternaria triticina spore suspension was made from pure culture of the pathogen grown on PDA. One drop of the spore suspension was placed on a slide and morphological characters were examined under compound microscope (Figure 1).
Characteristics of pathogen (Alterneria triticina )
• The mycelium of this fungus is hyaline. But later on it become alive buff in appearance.
• The conidiophores are septets usually unbranched, erect, single or in groups emerging out through the stomata.
• The conidiophores were light brown and septate.
• The conidia were usually yellowish brown in colour.
• The conidia may either be in chain or borne on conidiophores.
Description of conidium
• Colour: light brown to olive and become darker with age
• Shape: Irregularly oval, ellipsoid conical, gradually tapering into a beak
• Dimensions: 15-92 × 8-35 μm
• Septa: 1-10 transverse and 0-5 longitudinal.
Effect of bio-agents, neem leaf extract and fungicides against on mycelia growth of Alternaria
Antagonistic activity of Trichoderma viride, Trichoderma harzianum and Pseudomonas fluorescens and were investigated by dual culture method on PDA. Data reveals that, Trichoderma viride, Pseudomonas fluorescens were potential antagonists of Alternaria triticina forming a clear zone of inhibition. On microscopic examination hyphae of antagonists were observed coiling and oppressed around hyphae of Alternaria triticina, T. harzianum (85.50%) was most effective over other treatments followed by Trichoderma vridie (83.30%) and Pseudomonas fluorescens (80.73%) were least effective (Table 2 and Figure 2).
The efficacy of different fungicides and neem leaf extracts against Alternaria triticina were assayed in vitro . Observations were recorded on growth of the test fungus. Under various treatments, the observations recorded on per cent inhibition of growth are presented in Table 2 and Figure 2 that all the treatment at all the concentration inhibited the fungal growth and the data also revealed that the all the fungicides and neem leaf extracts were significantly superior over check at all the treatment propicanazole, hexaconazole, vitavax and neem leaf extract gave complete growth inhibition of Alternaria triticina.
| Treatments | Radial growth of Pathogen (cm) | Per cent inhibition (%) |
|---|---|---|
| Control | 5.45 | 0 |
| Propiconazole | 0.56 | 89.72 |
| Hexaconazole | 0.63 | 88.44 |
| Vitavax | 0.67 | 87.7 |
| Trichoderma viride | 0.91 | 83.3 |
| Pseudomonas fluorescens | 1.05 | 80.73 |
| Trichoderma harzianum | 0.79 | 85.5 |
| Neem leaf Extract | 1.44 | 73.57 |
| Mean | 11.5 | |
| F-test | s | |
| SEd (+) | 0.1 | |
| CD (5%) | 0.21 |
Table 2: Effect of bio-agents, neem leaf extract and fungicides against on mycelia growth of Alternaria.
A significant difference in data presented on inhibition per cent of mycelium growth was observed among the treatment. Maximum inhibition per cent was recorded on propicanazole (89.72%) except at it was followed by hexaconazole (88.44%), vitavax (87.70%) and neem leaf extract (73.57%).
Besides the agricultural practices, physical and biological methods used for the management of diseases caused by Alternaria triticina, chemical fungicides are most commonly adopted by the growers. Fungicides like; Propicanazole, carbendazim, hexaconazole, ridomil and topsin etc., have been recommended against Alternaria. Such synthetic fungicides bring about the inhibition of pathogens either by destroying their cell membrane or its permeability or by inhibiting metabolic processes of the pathogen and hence are extremely effective.
Effect of fungicides on the disease intensity of leaf blight of wheat
BAU-Biofungicide and botanical differed in respect of leaf blight disease Intensity (%) at different growth stages (Table 3). At 45 DAS, the lowest (16.18%) disease intensity was recorded with Propiconazole @0.1% Follwed by Hexaconazole @0.5% (17.92%). The highest disease incidence was recorded in control (33.00%), followed by Pseudomonas fluorescens fluorescens (19.15%), vitavax @0.25% (22.13%) Trichoderma viride (22.26%), Trichoderma harzianum (23.03%) and neem leaf extract @10% (23.50%). At 90 DAS, the lowest (18.24%) disease intensity was recorded with Propiconazole @0.1%. While the highest (43.18%) was recorded in control plot. Among the BAU fungicides and botanicals Propiconazole @0.1% and Pseudomonas fluorescens , performed better than other BAU fungicides and botanicals to reduce per cent disease intensity of the leaf blight disease (Table 3 and Figure 3).
| Treatment (%) | PDI | Yield (q\ha) |
C:B Ratio |
|
|---|---|---|---|---|
| 45 DAS |
90 DAS |
|||
| Control | 33 | 43.18 | 20.41 | 01:01.0 |
| Propiconazole @0.1% | 16.18 | 18.24 | 37 | 01:01.4 |
| Hexaconazole @0.5% | 17.92 | 19.94 | 34.63 | 01:01.4 |
| Vitavax @0.25% | 22.13 | 24.55 | 32.2 | 01:01.5 |
| Trichoderma viride @2% | 22.26 | 25.51 | 28.89 | 01:01.4 |
| Pseudomonas fluorescens @2.5% | 19.15 | 20.51 | 30.44 | 01:01.4 |
| Trichoderma harzianum @2% | 23.03 | 25.76 | 27.04 | 01:01.3 |
| Neem leaf extract @10% | 23.5 | 28.48 | 26.37 | 01:01.2 |
| S.Ed. (+) | 0.78 | 0.73 | 0.5 | - |
| C.D. (P=0.05%) | 1.67 | 1.56 | 1.07 | - |
PDI=Per cent disease intensity
DAS=Date after sowing
C:B=Cost Benefit ratio
Table 3: Effect of bio-agents and plant extracts on disease intensity of wheat.
Effect of BAU-fungicides and botanicals on the yield of leaf blight of wheat
Among the bio-agents, botanical and fungicides used the maximum grain yield was recorded in Propicanazole @0.1% (37.00) as compared to untreated control (20.41) followed by Hexaconazole @0.5% (34.63), vitavax @0.25% (32.20), Pseudomonas fluorescens flourescens (30.44), Trichoderma viride (28.89), Trichoderma harzianum (27.04) and neem leaf extract @10% (26.37) (Table 3).
Among the treatments most effective was propicanazole @0.1% (37.00) and hexaconazole @0.5% (34.63), However, the treatments neem leaf extract and Trichoderma harzianum were non-significant and statistically at par with each other [64]. The results of the present study are in accordance to the findings of the Nargund, they reported that in the present result showed that all the treatments tested in this study gave satisfactory result against Alternaria triticina.
Among all the treatment, systemic fungicides such as propicanazole and hexaconazole reduced that Alternaria triticina intensity and leaves become disease free [65]. Healthy leaves have more photosynthetic activity, ultimately enhance the number of ears. But the efficacy of bioagent such as Pseudomonas fluorescens, Trichoderma viride and Trichoderma harzianum were less as compared to the systemic fungicides [51].
Results showed that the highest yield was recorded in Propicanzole (37.00 q/ha), followed by Pseudomonas fluorescens flourescens @0.5% (30.44), reported that use of chemical inducers had adverse effect on the plant growth. But given highest yield because chemicals attributed to elicitor’s effect on physiological processes in plant such as ion uptake, cell elongation, cell divition, enzymatic activation and protein synthesis [66].
All treatments are significant to each other and statistically at par with each other. When cost benefit ratio was worked out, interesting result was achieved. Among the treatment studied, the best and most economical treatment was vitavax @0.25% (1:1.52), Trichoderma viride (1:1.43), propicanazole @0.1% (1:1.42), Hexaconazole @0.5% (1:1.36) followed by Pseudomonas fluorescens (1:1.38), Trichoderma harzianum (1:6.48), and neem leaf extract (1:1.22), as compared to control (1:1.03).
The application of Trichoderma viride reduces the pathogen population in soil by means of mycoparasitism and production of antibiotic which may be reduce the soil borne pathogens in soil. The inhibition of fungal growth due to Trichoderma spp. may have been due to secretion of extracellular cell degrading enzymes such as chitinase B-1, 3-glucanase, cellulose and lectin, which may have helped mycoparasites in the colonization of their host. The inhibition of pathogen may also be attributed to the production of secondary metabolites by antagonists such as glioviridin, viridian and gliotoxin [67,68].
All the plant extracts and BAU-Biofungicide significantly inhibited mycelial growth of the pathogen. BAU-Biofungicide showed maximum (89.72%) reduction of mycelial growth and based on the results, Pseudomonas fluorescens @2.5% was found the most effective treatment which gave recorded minimum disease intensity (%) and yield (q/ha), as compared to other treatments except Tilt @0.1% (propiconazole) which was taken as treated control. so it may be concluded that bio-agents along with propiconazole @0.1% can be use for the management of leaf blight of wheat. The present research findings are limited to one crop season (December 7- April 11) under Allahabad agro-climatic conditions as such more trials are required in future to validate the findings.
This manuscript is the part of M.Sc. (Ag) thesis work. Hence, the authors would like to thank the Department of Plant Pathology, SHUATS Allahabad, for providing the necessary facilities.