Research Article - (2016) Volume 4, Issue 1
The objective of this study was the evaluation of phytochemical, chemical composition, antioxidant and antimicrobial screening parameters in leaf of Rhamnus prinoides which are used to impart bitterness to local alcoholic beverages in Ethiopia and as traditional medicine in some African countries. The phytochemical screening of aqueous and methanol/water plant extracts was performed using standard phytochemical techniques for the determination of the presence of alkaloids, steroids, triterpines, tannins, flavanoids, flavones, phenols, glycosides, anthraquinones, proteins and resins. The nutritional value of samples were analyzed for their chemical composition (moisture, proteins, fat, carbohydrates and ash) using AOAC (Association of Analytical chemists procedures. The antimicrobial activities of the plant extracts against Neisseria gonorrhea, Staphylococcus aureus, Escherichia coli, Bacillus cereus, Pseudomonas aeroginosa, and Streptococcus fecalis using broth dilution technique were determined. These were compared with those of benzyl penicillin, oxytetracycline and streptomycin as positive controls in the experiment the extract of Rhamnus prinoides was positive for tannins, triterpenoids, cardiac glycosides, phenolics compounds, saponins and resins. Alkaloids were present in the methanolic/water extract only. The methanolic/water extract of Rhamnus prinoides also shows a broad spectrum of activity against all microorganisms tested except for Neisseria gonorrheae. This shows it has a high content of protein and fiber and is low in fat. The qualities demonstrate that it is this good alternative to other food stuffs and these results serve as the basis of further scientific study into various ways of enhancing the livelihood of particular areas of northern Mekelle through increased Rhamnus prinoides domestication as well as assessing the possible bioactivity of rhamnus against certain human diseases.
Keywords: Rhamnus prinoides; Phytochemical; Chemical composition; Antimicrobial; Mekelle; Methanol; Water
According to Kokwaro, Beentje and Hong et al. [1-3] among others Rhamnus prinoides belongs to the family Rhamnaceae and is commonly known as dog wood orshiny leaf. The plant is a shrub or a small dense thick bushy tree that may reach up to 9 m in height. The plant is common at medium to high altitude, along water courses, in riverine forest and at the margins of evergreen forests and frequently among rocks. It can be found in South African countries, tropical Africa, Kenya and Ethiopia. The plant thrives in moist humus soils.
The plant can be used as food as the fruits are edible. A decoction of the tree is taken as a blood purifier, treats pneumonia, gonorrhea, rheumatism and stomach ache. The leaves are applied as liniment to simple sprains. Further the tree can be cultivated to control soil erosion, it can be used as a hedge to act as a wind breaker and around fish ponds to protect and shade the fish. The plant can be used effectively as an ornamental plant (Figure 1). In Ethiopia, R. prinoides (gesho) can be found growing in the wild in all provinces, usually at altitudes of 1500- 2500 m. In most provinces, gesho is cultivated as well. It is present in many gardens near houses and it is sometimes cultivated as a field crop on a larger scale. In markets, fresh leafy branches are often sold. According to the Land Utilization Report, gesho covers approximately 1% (=5000 ha) of the total land area under permanent cultivation in Ethiopia. The most important provinces for gesho are: Shoa (1800 ha), Gojam (1200 ha), Begemdir (1000 ha), Arussi (500 ha), Sidamo (400 ha).
The species has a wide distribution in Africa. It occurs from Ethiopia to South Africa in the east and to Nigeria and Angola in the west [4-9].
In Ethiopia, gesho is used as a laxative, as a diuretic, as a preventive for syphilis, as a depurative and as a cholagogue. For children with tonsillitis or with tonsils removed (a common practice because the tonsils are considered responsible for 'sickness' in general), some macerated leaves of gesho are put in the mouth to relieve the pain [10-15]. In South Africa, a decoction of the decorticated root is taken to cleanse the blood by the Zulu and to treat pneumonia by the Sotho. The leaves are applied as a liniment to simple sprains by the Zulu. An extract of the root, together with the bark of Erythrinato mentosa is used by the Chagga to relieve colic, and the extract alone also for relief of muscular rheumatism. The root is a Masai remedy for gonorrhoea [16-20]. In Angola, the bark is used to induce vomiting [4]. The purpose of the current study was the evaluation of phytochemical, chemical composition, antioxidant and antimicrobial screening parameters Rhamnus prinoides (GESHO) cultivated in Tigray, Ethiopia.
Preparation of Rhamnus prinoides
The plant root materials were dried in the shade at room temperature for three weeks. The dried plants were then chopped into small pieces using a sharp knife and were ground into powder using an electric mill. The powder was packed into clean airtight polythene paper bags in portions of 500 grams in a fume cupboard. The workers wore face masks as added protection from the powder.
Preparation of aqueous extract of Rhamnus prinoides
Aqueous extractions were carried out according to the methodology of Erazo et al. and Gakuya [21,22]. Forty grams of plant powder was weighed into a conical flask and dissolved in 400 mls of distilled water. The mixture was boiled in a hot water bath (100°C) for 30 minutes. The resultant extract was filtered through muslin gauze into clean vials then centrifuged at 4000 revolutions per minute for ten minutes. The supernatant was transferred to clean vials and lyophilized for 24 hours in a lyophilizer (Edwards High Vacuum, Model M6B) [23-26].
Preparation of methanol/water extract of Rhamnus prinoides
Forty grams of powder was weighed into a conical flask and soaked in methanol 70% v/v methanol /water. The mixture was macerated for three days with continual shaking. The extract was then filtered using Whatman No. 1 filter paper, and was then evaporated to dryness in a rotary evaporator under vacuum. The resultant residue was put in an oven at 40°C for methanol to be reduced completely. The final residue (3.62 gms of yield) freeze dried for 24 h then lyophilized [27-30].
Phytochemical screening
Phytochemical screening of the extracts of Rhamnus prinoides (Hoechst) Vatke was carried out according to standard methods of Harborne.
Test for alkaloids: Half a gram (0.5 g) of plant extract was weighed and stirred in 2 ml of 1% aqueous hydrochloric acid and heated in a boiling water bath for 10 minutes. The mixture was filtered while hot and treated with Dragendorff‘s reagent. Turbidity or precipitation indicated the presence of alkaloids.
Test for sterols and triterpenes: Half a gram (0.5 g) of the extract was defatted with hexane. The residue was then extracted in dichloromethane and the solution dehydrated with magnesium sulphate anhydride. The mixture was treated with 0.5 ml acetic anhydride followed by addition of 2 drops of concentrated sulphuric acid. A gradual appearance of green blue colour indicated presence of sterols while colour change from pink to purple indicated the presence of triterpenes [31-35].
Test for saponins: Half a gram (0.5 g) of the plant extract was dissolved in 5 ml of distilled water and shaken for at least five minutes. Frothing that persisted for at least half an hour was used to indicate the presence of saponins.
Test for flavonoids and flavones: Two hundred milligrams (200 mg) of the extract was dissolved in 4 ml of 50% methanol. The solution was warmed and metal magnesium added. Five drops of concentrated suphuric acid were then added. Development of a red colour indicated the presence of flavonoids while orange colour showed presence of flavones.
Test for tannins: Screening for tannins was done using both ferric chloride and lead acetate tests. For the ferric chloride test, half a gram (0.5 g) of the extract was dissolved in 2 ml of distilled water and filtered. Two drops of ferric chloride were then added to the filtrate. Development of a blue-black precipitate indicated that tannins are present. For the lead acetate test, in a test tube containing about 5 mg of extract, a few drops of 1% solution of lead acetate were added and the formation of a yellow or red precipitate indicated the presence of tannins.
Test for cardiac glycosides: The Keller Killian test was applied. A hundred milligrams (100 mg) of extract was dissolved in 1 ml of glacial acetic acid containing one drop of ferric chloride solution. This was then under-layered with 1 ml of concentrated sulphuric acid. The appearance of a brown ring at the interface of the two layers with the lower acidic layer turning blue green upon standing indicated the presence of cardiac glycosides [36-40].
Test for resins: To 2 mg of plant extract, 5 to 10 ml of acetic anhydride was added and dissolved by gentle heating. After cooling, 0.5 ml of sulphuric acid was added. Bright purple colour produced indicated the presence of resins.
Test for anthraquinones: One gram (1 gm) of the extract was dissolved in 70% acetone to a final concentration of 50 mg/ml. The Bonträger Test was used to test for anthraquinones. Two milliliters (2 ml) of the test sample was shaken with 4 ml of hexane to defat. The upper lipophilic layer was separated and treated with 4 ml of dilute ammonia. The change of the lower layer to violet and then pink indicated the presence of anthraquinones.
Test for phenols (Ferric Chloride Test): 2 ml of distilled water was added to 1 mg of plant sample followed by a few drops of 10% aqueous ferric chloride solution. Formation of blue or green colour indicated the presence of phenols.
Test for glycosides: 2 mg of plant extract sample was dissolved in 1 ml distilled water and then aqueous sodium hydroxide was added. Formation of a yellow colour indicated the presence of glycosides.
Disc diffusion method
The antimicrobial activity of the prepared extracts was determined by using disc diffusion method [27]. The inoculated extracts were then examined for inhibition zones (in mm) by zone reader, which indicates antimicrobial activity The discs (6 mm in diameter) were impregnated with 20 μg/m, sample extracts (20 μg/disc) and placed on inoculated agar. Rifampicine (20 μg/disc) (Oxiod) and Fluconazole (20 μg/disc) (Oxiod) were used as a positive reference for bacteria and fungi, respectively [41-50].
Determination of minimum inhibitory concentration (MIC)
The medium used in this assay was Isosensitest and standardized bacterial colony numbers was taken, adopting 5 × 105 CFU/mL final concentration of strains of bacterial species. After preparing microbial culture and resazzurin solution glass plates were prepared. These were all prepared under aseptic conditions. A 96 well plate was sterilized and labeled. 100 μL of test material in 10% (v/v) DMSO/sterile water (10 mg/mL for crude ex-tracts) was pipetted into the first row of the plate. In the rest of the wells 50 μL of nutrient broth was added and serial dilutions were made by adding 50 μL of the test sample in serially descending concentrations. In each well 10 μL of the resazzurin indicator solution was added. At the end 10 μL of bacterial suspension (5 × 106 CFU/ mL) was added to each well to get a concentration of 5 × 105 CFU/mL. To avoid bacteria to become dehydrated each plate was wrapped loosely with cling film. Every plate had a set of control column having broad-spectrum antibiotic as positive control (Ampicillin for bacteria: Benzyl penicillin for fungi). That column has all solutions except the test compound, and another column with all solutions with the exception of the bacterial solution adding 10 L of nutrient broth instead. The plates were prepared in triplicate, and placed in an incubator set at 37°C for 18-24 h. The color change was then assessed visually. Any color changes from purple to pink or colorless were recorded as positive. The lowest concentration at which color change occurred was taken as the MIC value. The average of three values was calculated and that was the MIC for the test material and microbial strain and the physiochemical properties of the plant can be determined using standard methods of AOAC (20).
Chemical composition
The samples were analyzed for chemical composition (moisture, proteins, fat, carbohydrates and ash) using the AOAC procedures (2). The leaves of the plant were dried for the estimation of ash, proteins, fiber, fat and total carbohydrates.
Determination of total ash: About 3 grams of sample is weighed in a crucible and as heated in a muffle furnace at 550 degree Celsius for 30 minutes and cooled in desiccators. The ash content was calculated using following equation.
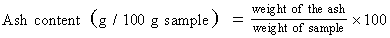
Determination of total proteins: To about 0.7 gram of sample in a digestion flask, 1 gram of Copper Sulphate, 10 grams of Potassium sulphate and 20 ml of Sulphuric acid was added. After complete digestion the content was transferred into a vessel. 25 ml of 0.2N Sulphuric acid was pipetted out into beaker and distillation was started. The distillate was allowed to collect in a known volume of Sulphuric acid for over a known length of time. The collected distillate was titrated against 0.2N Sodium Hydroxide using Methyl red as an indicator.
The percentage of Protein was calculated.
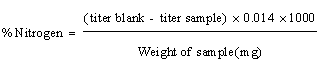
% of Protein=% of Nitrogen × 6.24
Determination of fat content: About 10 grams of Rhamnus prinoides sample was weighed and extracted with Petroleum Ether in an extraction apparatus for 16 hours. The extract was dried, cooled in desiccators, weighed and the mass was recorded. The % of fat was determined using an equation
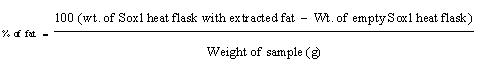
Determination of fiber content: 5 grams of Rhamnus prinoides sample was extracted using Petroleum ether. The fat free material was transferred in a beaker and 200 ml of dilute sulphuric acid was added and boiled. Whole boiling acid in a flask is connected to reflux condenser and heated for 30 minutes. The flask was removed and filtered and washed thoroughly with boiling water followed by washing in boiling Sodium Hydroxide and again refluxed for 30 minutes. The contents were filtered and washed with boiling water and finally washed in ethanol. The residues were dried and incinerated in muffle furnaces at 660°C and the crucible along with ash was weighed and percentage of fiber was calculated.
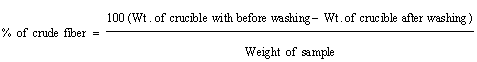
Determination of total carbohydrates: The percentage of total carbohydrates was calculated by the difference method according to this equation:
(100 - Total moisture + Total ash + Total moisture + Total protein + Total fat + Total fibers) the percentage of carbohydrates was calculate.
Both aqueous and methanol/water extract showed similar phytochemicals with the exception of methanol/water that showed the presence of alkaloids. The chemicals included triterpenes, saponins, tannins, glycosides, phenols, cardiac glycosides and resins (Table 1).
| Type ofphytochemical | Aqueousextract | Methanol/Water extract |
|---|---|---|
| Alkaloids | - | + |
| Steroids | - | - |
| Triterpene | + | + |
| Saponins | + | + |
| Tannins | + | + |
| Flavonoids | - | - |
| Flavones | - | - |
| Phenols | + | + |
| Glycosides | + | + |
| Cardiac glycosides | + | + |
| Anthraquinones | - | - |
| Resins | + | + |
+: Presence of phytochemical; -: Absence of phytochemical
Table 1: The phytochemicals present in the aqueous and methanol/water extracts of Rhamnus prinoides growing in Tigray, Ethiopia.
The phytochemical analysis of the medicinal plant extract showed similar chemicals except that alkaloids were present in both aqueous and methanolic extracts. Alkaloids were additionally present in the methanolic extract. The phytochemicals present included; Tannins which are polymeric phenolic substances capable of tanning leather or precipitating gelatin from solution, a property known as astringency [51-53]. They are used in pharmaceutical preparations because of their astringent action. Tannins are also known to possess general antimicrobial and antioxidant activities [54]. At low concentration, tannins can inhibit the growth of microorganisms, and act as an antifungal agent at higher concentration by coagulating the protoplasm of the microorganisms [55,56]. Tannins may have potential value as cytotoxic and antineoplastic agents [57] but are now being used in the manufacture of plastics, paints, ceramics and water softening agents [58]. Their presence in the extracts under study hence validates the use of the plant as a medicinal remedy by the Samburu community.
Triterpenoids are known for their anti-inflammatory, hepatoprotective, analgesic, antimicrobial, antimycotic, virostatic, immunomodulatory and tonic effects. They are used in the prevention and treatment of hepatitis, parasitic and protozoal infections and for their cytostatic effects. These properties qualify the herbal remedies use for treatment of Sexually Transmitted Infections and Infertility as reported by the traditional healers. The disadvantage of triterpenoids is the toxicity associated with their hemolytic and cytostatic properties and therefore with ongoing extraction and isolation of natural products there is need to develop their synthetic derivatives with lower toxic and higher therapeutic potential [59]. In the present study the plant has been reported to have toxic effects especially with high dose consumption, and hence is recommended to be administered only by an experienced traditional healer. Further work is necessary to isolate and identify the active constituents of the plant and elucidate the mechanism of their toxic effects as well as determine the correct dosage that would be safe for future prescriptions.
Cardiac glycosides are known to work by inhibiting the sodium and potassium pump (Na+, K+, ATPase). This causes an increase in the level of sodium ions and the calcium ion. This inhibition increases the amount of Calcium ions available for contraction of the heart muscle which improves cardiac output and reduces distention of heart; thus are used in the treatment of congestive heart failure and cardiac arrhythmia [60,61].
Phenolic phytochemicals were present and have been reported to have antioxidative, antidiabetic, anticarcinogenic, antimicrobial, antimutagenic and anti-inflammatory activity [62-65]. Such activities would qualify the plant for use as a medicinal remedy.
Saponins which were present have been reported to have mild detergent activity and are used in intracellular histochemistry staining to allow antibody access to intracellular proteins. They are also used in hypercholesterolemia, hyperglycemia, antioxidant, anticancer, antiinflammatory and weight loss, among other factors. They are also known to have anti-fungal properties [66] and have been implicated as bioactive antibacterial agents of plants [67,68].
Alkaloids were present in the methanolic extract and are plant metabolites with alkali-like chemical reactivity, and pharmacologic activity. They represent a very diverse group of medically significant compounds such as the opiates. Ergot alkaloids have been used in the treatment of migraine, as uterine contractions and vasoconstrictor agents [69,70]. In addition they exhibit antibiotic activity, antimalarial activity as well as anticancer activity. Their presence in this plant remedy validates its use as the traditional healers claim.
It has been reported that resins are secreted by plants as secondary plant metabolites. The thick and sticky substance apart from being used in paints, are also used in medicine. A variety of trees produce them and each variety has a slight difference in the resins they secrete. Resin in frankincense has been used by the Egyptians in the olden times as eyeliner for its healing properties and recently as a pill for various diseases. Because of their anti-inflammatory properties resins have been used in treatment of arthritis as well as in aroma therapy to release stress and anxiety in Africa (http://www.arthritistoday.org).
The presence of the various phytochemicals in the crude extracts which have various pharmacological activities could explain why the Samburu community uses the plants extensively. The phytochemical composition of the same plant from a geographically different area, the Nandi South District of Kenya was different. This might be explained by the difference in the soils of the two areas, the time of harvest, the mode of extraction, the mode of storage among other factors [1]. This implies that the use of herbal remedies would be affected by geographical variation, a fact that requires further investigation to validate [71-73].
The minimum inhibition concentration of selected medicinal plants
Rhamnus prinoides aqueous extract was not active against any of the bacterial strains that were tested in this work but its methanol water extract was active against all bacteria strains except Proteus vulgaris, Bacillus subtilis and Staphylococcus epidermidis generally. The concentration of the extract of the medicinal plants used that showed antimicrobial activity was observed to be high. The details of the selected medicinal plants, the bacterial strains used, the concentration of the extracts used and are listed in Table 2.
| Name of Microorganism | Minimum inhibitory concentration(mg/l) | |
|---|---|---|
| Methanolextract | Water | |
| Escherichia coli | 400 | - |
| Staphylococcus aureus | 200 | - |
| Proteus vulgaris |
|
- |
| Pseudomonas aeroginosa | 400 | - |
| Bacillus subtilus | - | - |
| Staphylococcus epidermidis | - | - |
| Streptococcus mutans | 200 | - |
Table 2: Rhamnus prinoides minimal inhibitory concentrations of plant extracts against bacterial isolates (mg/ml).
Rhamnus prinoides which has antibacterial activity has also been reported to treat venereal diseases. This fact can be investigated further in the case of the plants used in this study and especially those reported to perform best when in combination. This may improve the concentration of the plant extract that show antimicrobial activity. It is also noted that a higher concentration of the plant extract is used for activity against bacteria strains used. This may suggest that the concentration of the noble molecule within the extract was low. The antimicrobial activity of the medicinal plant extract against the bacterial strains used was not statistically significant (P>0.05). The method of extraction together with the method of storage may also have affected the quantity of active molecule available in the extract. All the selected plants showed some antimicrobial activity which differed between the aqueous or methanol/water extract. Gram negative bacteria usually require a higher concentration of extract for activity than do the gram positive bacteria. This observation could be attributed to the cell wall of gram positive bacteria which is easier to penetrate than that of gram negative bacteria. This fact may explain why the gram negative bacteria in the present study showed a higher MIC than the gram positive bacteria. Most extracts did not show antibacterial activity even though their medicinal use has been reported in literature. This fact could be explained by the fact that many herbal medicines act as pro drugs and their activity will be seen in vivo and not necessarily in vitro. Further the herbal preparations may be subject to contamination and deterioration. This study clearly shows that the herbal remedies under study have antimicrobial activity which validates their use by the traditional healers (Table 3).
| Content | Units |
|---|---|
| Moisture | 9.5 g |
| Nitrogen | 1.6 g |
| Protein | 8.5 g |
| Fat | 3.5 g |
| Carbohydrate | 70.5 g |
| Fibre | 25.6 g |
| Ash | 9.5 g |
Table 3: Nutritional analysis of cultivated Rhamnus prinoides (% in grams).
The proximate and nutrient analyses of Rhamnus prinoides play plays a crucial role in assessing its nutritional significance (<0.05). The proximate analysis for the mean moisture, lipid, fiber, and ash values found in the present study are in agreement with the values reported with standard of WHO [28]. The result shows that Rhamnus prinoides found in the market of Mekelle contain has this nutritional composition: crude protein (8.5%), crude fiber (about 25.6%), ash (about 9.5%), carbohydrates (about 70.5%), moisture (about 9.5%), and lipid (about 3.5%). Carbohydrates are the principal sources of energy. The ash content of about 9.5% indicates that the leaves are rich in mineral elements. The mean protein content found in the market of Mekelle samples ranged from 8.5%. The chemical composition values confirmed that the powder made from Rhamnus prinoides leaves are an excellent food source, justifying their direct use in human nutrition or development of balanced diets for animal nutrition.
Extracts of Rhamnus prinoides obtained from Mekelle, Tigray contain several phytochemicals that include alkaloids, triterpene, saponins, tannins, phenols cardiac glycosides and resins. These phytochemicals may be responsible for the pharmacological and a toxicology action of the plant which has antibacterial activity has also been reported to treat venereal diseases. The chemical composition values confirmed that Rhamnus prinoides leaf powders are an excellent food source, justifying its direct use in human nutrition or development of balanced diets for animal nutrition.