Research Article - (2018) Volume 6, Issue 5
Keywords: FAJ; NFAJ; Antioxidant; Anti-inflammatory; TNF-α; MAPK pathways
Reactive oxygen species (ROS), consisting of radical and nonradical oxygen species, such as hydrogen peroxide (H2O2), superoxide anion (O2), and hydroxyl radical (•HO) induce damage to cellular components in human body and causes chronic illnesses [1,2]. Antioxidants, having chelating effect against free radicals, play important roles in preventing cellular oxidation and the oxidationrelated diseases [3]. While the synthetic antioxidants are known as efficient free radical scavengers, they are often associated with adverse side effects. As a consequence, antioxidants derived from natural sources are gaining attention in preventing oxidative stress-related diseases [3,4].
It is well-known that oxidative stresses induce damage to the immune system and causes inflammation [5]. Inflammation is a part of the immune response that acts against pathogens and detrimental stimuli and help protect our body [6]. This response is launched as soon as immune cells detect any pathogen invasion [5,6]. After being triggered by cellular signals, macrophages accelerate the immune response by releasing pro-inflammatory cytokines and mediators in the connective tissues to fight against pathogens [7]. However, uncontrolled inflammatory response by macrophage cells can lead to chronic inflammatory diseases [8]. Previous reports indicate that LPSstimulated macrophage cells release inflammatory cytokines and mediators, including TNF-α, nitric oxide (NO), and prostaglandin E2 (PGE2) [9,10]. Among various inflammatory pathways, mitogenactivated protein kinase (MAPK) family, which consists of p38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), regulate the inflammation process, including the activation of inflammatory cytokines [11,12]. Synthetic steroids are generally prescribed as primary treatment for chronic inflammation; however, steroids are supposed to weaken the immune system by disrupting the cytokine network [13,14]. Therefore, effective natural therapeutic interventions that can target the intraplaque macrophages and their released products can be suggested for preventing inflammatory diseases.
Achyranthes japonica Nakai (AJ) has been popularly used as traditional medicine in East Asia for the treatment of chronic diseases [15]. AJ contains bioactive compounds, including inokosterone, ecdysterone, oleanolic acid, bisdesmoside and saponins, which are known to possess scavenging effects against free radicals [16,17]. Reports suggest that AJ extract has potent activities against inflammation and osteoarthritis owing to its bioactive compounds [15]. Fermentation is a novel process that can increment the biological activity of natural extracts through the enrichment of active compounds using bacterial strains [18]. This process has gained wide applications in food and pharmaceutical industry [19,20].
Therefore, the present study was carried out to investigate the antioxidant and anti-inflammatory properties of the fermented AJ extract with the underlying mechanisms.
Chemicals and reagents
Bacillus subtilis KCCM 10835P were obtained from Korea Culture Center of Microorganisms (Seoul, Korea). The antibodies for pp38, p38, ERK, pERK, JNK, and anti-pJNK, were purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA, USA). Fetal bovine serum (FBS), streptomycin, trichloroacetic acid (TCA), 3-(4,5 dimethythiazol2- yl)-2,5-diphenytetrazoleum (MTT), Dulbecco’s Modified Eagle’s medium (DMEM), and penicillin were obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan). All other chemicals and reagents used in this study were of analytical grade and obtained from Sigma Aldrich Inc. (St. Louis, MO, USA).
Extraction and fermentation of AJ
Dried AJ was mechanically ground into powder form. The powder (100 g) was extracted three times with 75% ethanol. FAJ was fermented using a previously described method [16]. Briefly, AJ was mixed with distilled water (DW) and autoclaved at 120°C for 20 min. The solution was then fermented with 5% Bacillus subtilis KCCM 10835P at 40°C for 7 days. Both fermented and non-fermented extracts were filtered through Whatman No. 1 filter paper (GE Healthcare UK Limited, Buckinghamshire, U.K.) followed by the evaporation under vacuum condition. The crude extracts of AJ were freeze-dried at -80°C and stored at -20°C. The obtained yields of NFAJ and FAJ were 12.08 and 28.86% (w/w), respectively.
Measurement of phenolic and flavonoid contents
Total phenolic content was measured according to a method described earlier [21]. Each sample (1 mg/ml) and different concentrations (0.0078-1 mg/ml) of gallic acid were prepared in a 96 well-plate. An aliquot of 40 μl of AJ extracts or standard were mixed with 20% Na2CO3 (60 μl, w/v) and 1 M FC reagent (20 μl) and stored in a dark place at RT for 30 min for reaction. The absorbance of the mixture was detected at 700 nm by UV- spectrophotometry (Sunrise Basic Tecan, Austria). The obtained data was expressed as standard equivalents (gallic acid (GAE)/100 g dry mass). Total flavonoid content was measured using a previously described method [22]. A volume of 25 μl of AJ extracts (1 mg/ml) and catechin (standard) with different concentrations (0.05 mg/ml to 0.5 mg/ml) were mixed with DW (125 μl) in 96 well-plate. 5% NaNO3 (8 μl) was then added and the mixture was kept on a shaker for 5 min. Then, 10% AlCl3 (15 μl) was added to the mixture and kept with shaking for 6 min. The reaction was stopped by adding 0.1 M NaOH (50 μl) and D.W. (27 μl). After pipetting, flavonoid content was detected at 517 nm. The calculated data was expressed as standard equivalents (catechin (CE)/100 g dry mass).
DPPH radical scavenging activity
This assay was carried out following a method mentioned earlier [23]. In this method, the hydrogen donating ability of the extract was determined based on the changing of a radical methanolic DPPH solution into non-radical form. In short, different concentrations (0.125-4 mg/ml) of AJ extracts and BHT (standard) were dissolved in DW and MeOH (100%), respectively. Then 80 μl of each sample or standard solution was mixed with 80 μl of 0.3 mM DPPH solution. The mixture was then kept at RT in darkness under vigorous shaking for 30 min. Finally, the absorbance was read at 517 nm using a microplate reader. DPPH radical scavenging activity was calculated using the below equation:
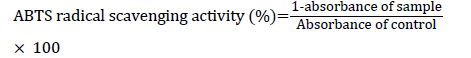
ABTS radical scavenging activity
ABTS radical scavenging activity was determined using a method described earlier [24]. Initially, a 7 mM ABTS solution was prepared in 2.5 mM potassium and stored in darkness at RT for 16 h to generate ABTS radicals. ABTS+ solution was diluted with 0.01 M phosphate buffered saline (PBS, pH 7.4), and the absorbance was adjusted to 0.70 ± 0.02 at 734 nm. A volume of 300 μl of AJ extracts or AA (standard) with various concentrations (0.250-4 mg/ml) were reacted with 700 μl of ABTS+ solution and kept at RT for 5 min for incubation. The absorbance was measured at 734 nm. ABTS radical scavenging activity was calculated using the following formula:
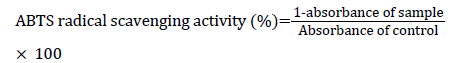
Nitrite scavenging activity
Nitrite scavenging activities of the samples were measured using the method of Cui et al. [25]. An aliquot of 1 ml of diluted AJ extract (0.250-4 mg/ml) or diluted AA (0.250-4 mg/ml) were added to 1 mM NaNO2 (1 ml). The mixture was added with citrate buffer (8 ml, pH 3) and incubated at 37°C for 50 min. The solution (1 ml) was then added with acetic acid (2 ml) and Griess reagent (4 ml). The solution was mixed with a vortex mixer and kept at RT for 15 min. The final solution was then detected at 520 nm. The scavenging activity of nitrite (%) was calculated using the following equation:
Reducing power activity
Reducing power activity was determined following a method mentioned earlier [26]. AJ extracts (1 ml) and AA (positive control, 1 ml) at different concentrations (0.250-4 mg/ml) were mixed with 0.2 M Na3PO4 (pH 6.6) and 1% C6N6FeK3 and kept at 50°C for 30 min. The solution was then added to 10% TCA (2.5 ml) and centrifuged (3,000 rpm). The supernatant (0.25 ml) was collected and mixed with D.W. (250 μl) and 0.1% FeCl3 (500 μl). The final mixture was detected at 700 nm.
Hydroxyl radical scavenging activity
This assay was conducted via electron spin resonance (ESR) technique (JES-FA100, JEOL Ltd., Tokyo, Japan) as described in a previous study [27]. Firstly, 10 mM H2O2 and 0.3 M DMPO were diluted using PBS buffer (pH 7.4). Various concentrations (0.250-4 mg/ml) of AJ extract (20 μl) or AA (20 μl) was then mixed with 10 mM FeSO4 (20 μl), H2O2 (20 μl), and DMPO (20 μl). The solution was detected through JES-FA100 spectrometry (JEOL Ltd., Tokyo). The chelating effect of this assay (%) was calculated as follows:
Hydroxyl radical scavenging activity=[(peak of control-peak of sample)/peak of control] × 100
Superoxide radical scavenging activity
In this method, an aliquot of 20 μl of AJ extract (0.250-4 mg/ml) or AA (0.250-4 mg/ml) was gently added to a mixture consisted of 1.6 mM EDTA (20 μl), 0.8 M DMPO (20 μl), and 0.8 mM C17H20N4O6 (20 μl)). The solutions were illuminated under a UV lamp (absorbance value: 365 nm) for 60 sec before detecting with JES-FA100 spectrometry (JEOL Ltd., Tokyo).
Cell viability assay
The macrophage (RAW 264.7) cells obtained from Korean Cell Line Bank (Seoul, Korea) were seeded in a 96 well plate. After incubation for 24 hours, the cells were treated with AJ extracts (0.250-4 mg/ml) and/or LPS (1 μg/ml), and incubated for 18 h. Then, MTT solution (0.2 mg/ml) was gently added to the plate and incubated for 4 h. Finally, 100 μl of DMSO was added to the solution and the absorbance was measured at 540 nm using a microplate reader (ELISA, Bio-Rad, Hercules, CA, USA).
Measurement of nitric oxide
Griess reagent method was used for the evaluation of nitric oxide (NO) production in RAW 264.7 cells [27]. Cells were seeded (5 × 104 cells/well) with DMEM medium in 96 well-plates. After 24 h of incubation, the cells were treated with different concentrations of AJ extracts (0.250- 4 mg/ml) dissolved in PBS (pH 7.4) with or without LPS (1 μg/ml) for 18 h. Then, 80 μl of cultured medium was mixed with 80 μl of Griess reagent and kept for incubation at RT in darkness under continuous shaking for 10 min. The absorbance was measured at 540 nm using a microplate reader. The concentration of NO was determined by comparing with a sodium nitrite (NaNO2) standard curve.
Measurement of PGE2 production
The macrophage cells were seeded (1 × 106 cells/ml) and treated (or untreated) with AJ extracts at various concentrations (0.500-4 mg/ml) for 30 min. After incubation, 1 μg/ml of LPS was added to the cultured media. After centrifugation at 5,000 × g for 10 min, the supernatant was collected and the PGE2 production in the cells was measured by an assay kit (Cayman Chemical, Ann Arbor, MI, USA).
Reverse-transcriptase polymerase chain reaction (RT-PCR) assay
Total RNA was isolated from macrophage cells using Trizol reagent (Life Technologies, CA, USA). Complementary DNA (cDNA) was reverse-transcribed using oligo (dT12-18, 0.5 μg/μl), MuLV reverse transcriptase, and 1 mM deoxyribonucleotide triphosphate (dNTP). The sequences of the primers were designed as follows: TNF-α: 5'- AGCACAGAAAGCATGATCCG-3' and 5'- CTGATGAGAGGGAGGCCATT-3'; GAPDH: 5'- GCAAAGTGGAGATTGTTGCCATC-3' and 5'- CATATTTCTCGTGGTTCACACCC-3'. PCR products were electrophoresed in an agarose gel (2%) stained with ethidium bromide. The quantification of the change of mRNA levels was performed using an Image J 1.46 software (NIH, Bethesda, MD, USA).
Western blot assay
The LPS-induced RAW 264.7 cells were treated with AJ extracts (1-4 mg/ml). Nuclear and cytoplasmic protein extraction was carried out according to the manual of NE-PER kit (Pierce Biotechnology, Rockford, IL, USA). Protein concentration of the supernatant was determined using Bradford reagent (Bio-Rad, Hercules, CA, USA). SDS-polyacrylamide gel electrophoresis was performed to separate and transfer the protein (50 μg) into a nitrocellulose membrane (Millipore, Billerica, MA, USA). The membrane was blocked using TBST solution and kept in diluted primary rabbit antibodies (1:1000) for 24 hours. After washing with TBST solution, the membrane was incubated with secondary rabbit antibodies (1:2000) at RT for 1 h. The immunodetection was enhanced using a chemiluminescent substrate (ECL, SurModics, MN, USA) and the immunosignals were captured by LAS-3000 system (Fuji Photo Film Co., Ltd., Japan). The intensity of the protein expression levels were analysed by Image J 1.46 software (NIH, Bethesda, MD, USA).
Statistical analysis
The obtained data were represented as mean ± standard deviation (S.D, n=3). All data were analysed using GraphPad Prism 5 (GraphPad software, Inc., La Jolla, CA) and Microsoft Excel 2010. The statistical significant was set at P<0.05 and the analyses were carried out using one-way analysis of variance (ANOVA).
Total phenolic and flavonoid contents
Polyphenols found in plant extracts have numerous bioactive functions [28]. Total phenolic contents of NFAJ and FAJ were 2.52 mg and 5.75 mg GAE per 100 g of dry mass, respectively (data not shown). The flavonoid contents of NFAJ and FAJ were 2.27 and 3.56 g CE per 100 g dry mass, respectively (data not shown). Interestingly, phenolic content in FAJ were twofold higher than NFAJ. The content of flavonoid was also significantly higher in FAJ compared to NFAJ.
Free radical scavenging activities of AJ extracts
Antioxidants scavenge free radicals, such as DPPH and ABTS by donating hydrogen. AJ extracts showed concentration-dependent scavenging activities against DPPH, ABTS, and nitrite radicals. DPPH radical scavenging activities of NFAJ and FAJ were 9.84-58.43% and 33.15-87.16%, respectively at the concentration of 0.125-4 mg/ml (Table 1). The inhibitory effect of NFAJ (36.31%) against DPPH was significantly lower than the positive control (95.36%), whereas the effect of FAJ (86.33%) was close to that of the positive control at 2 mg/ml concentration level. In addition, the half maximal inhibitory concentration (IC50) of FAJ (0.36 mg/ml) was significantly lower than NFAJ (3.02 mg/ml). Similarly, ABTS radical scavenging activity of FAJ (75.04%) was significantly (*P<0.05) higher than NFAJ (49.24%) at 2 mg/ml of concentration. Moreover, the IC50 value of FAJ (1.49) was lower than NFAJ (2.19). Nitrite scavenging activity was also remarkably higher in FAJ (37.33%) than NFAJ (21.76%). The IC50 values of NFAJ and FAJ were 4.19 and 3.07 mg/ml, respectively.
| Extract | DPPH radical | ABTS+ radical | Nitrite radical | Reducing power | |||
|---|---|---|---|---|---|---|---|
| Chelating activity (%) (2 mg/ml) |
†IC50value (mg/ml) |
Chelating activity (%) (2 mg/ml) |
†IC50 value (mg/ml) |
Chelating activity (%) (2 mg/ml) |
†IC50 value (mg/ml) |
††EC50value (mg/ml) |
|
| NFAJ | 36.31 ± 1.92a | 3.02 ± 0.05a | 49.24 ± 1.75a | 2.19 ± 2.34a | 21.76 ± 2.35a | 4.19 ± 0.13a | 1.66 ± 0.25a |
| FAJ | 86.33 ± 0.88b | 0.36 ± 0.09b | 75.04 ± 1.73b | 1.49 ± 2.34b | 37.33 ± 1.52b | 3.07 ± 0.11b | 0.59 ± 0.05b |
| Positive control |
95.36 ± 0.37c (BHT) | 0.02 ± 0.01c | 99.43 ± 1.28c (Ascorbic acid) |
0.01 ± 0.01c | 61.43 ± 1.42c (Ascorbic acid) |
1.42 ± 0.05c | 0.12 ± 0.01b |
All data represent as means ± S.D (n=3).
†The data-values of IC50 (mg/ml) means the concentration of each extract which provides 50% of inhibition effect in the DPPH, ABTS×+, and nitrite radical assays.
††The data-values of EC50 (mg/ml) means the concentration of each extract which provides 0.5 value of effective effect of absorbance in the reducing power experiment.
a-cThe denoted statistical significant was set at P<0.05, comparison to BHT or ascorbic (positive control).
Table 1: The chelating effect of DPPH, ABTS×+, nitrite radical with IC50 and EC50 (of reducing power) values of non-fermented (NFAJ) and fermented (FAJ) extracts of Achyranthes japonica Nakai.
Reducing power, hydroxyl and superoxide radical scavenging activities of AJ extracts
Reducing power activity, which determines the electron donating ability of the extracts, is shown in Figure 1A. Both NFAJ and FAJ showed concentration-dependent Fe (III)-reducing activities, and the activities of FAJ (0.10-1.18 nm) was higher than NFAJ (0.09-0.58 nm) at 0.125-4 mg/ml concentration. The EC50 values of NFAJ and FAJ, which measure the concentration at which 50% of the maximum effect is produced, were 1.66 and 0.59 ± 0.05 mg/ml, respectively (Table 1). The scavenging activities of the extracts against hydroxyl radical (•HO) are shown in Figure 1B. The results indicate that the hydroxyl radical scavenging activities of NFAJ and FAJ were dose-dependently increased, and FAJ (11.24-79.13%) demonstrated higher efficiency than NFAJ (14.23-48.14%) at 0.25-4 mg/ml concentration level. AJ extracts exhibited O2- scavenging effects, which ranged between 6.80 and 43.19%, and 9.30 and 48.63% for NFAJ and FAJ, respectively at 0.25-4 mg/ml concentration (Figure 1C). The above results suggest that the fermentation of AJ extract resulted in a significant increase in antioxidant activities.
Figure 1: Reducing power (A), Hydroxyl (B), and superoxide radical (B) scavenging activity of non-fermented (NFAJ) and fermented (FAJ) extracts of Achyranthes japonica Nakai. All data are expressed as mean ± SD (n=3). *Indicates significant differences compared with the positive controls BHT (A) and ascorbic acid (B, C and D) (p<0.05).
Effects of AJ extracts on cell viability and the release of NO and PGE2
Cytotoxic effects of the AJ extracts were measured in LPS-induced RAW 264.7 cells using MTT assay. As shown in Figure 2A, neither of the extracts produced any significant cytotoxic effects at 0.125-4 mg/ml concentration level. However, viability of the macrophage cells treated with FAJ was relatively higher than those treated with NFAJ. Macrophage cells release NO in response to host defense mechanism, the overproduction of which causes tissue damage and inflammation. NO production in the LPS-induced RAW 264.7 cells was dosedependently decreased with the treatment of NFAJ and FAJ (Figure 2B). FAJ produced stronger inhibitory effect on NO production compared with NFAJ. Prostaglandin E2 is regarded as in important mediator in the inflammation process. As shown in Figure 2C, PGE2 level in RAW 264.7 cells was elevated from 182.68 pg/ml to 1,105.68 pg/ml after being stimulated with LPS. The elevation of PGE2 level was markedly (*P<0.05) reversed by NFAJ (753.07 pg/ml) and FAJ (602.70 pg/ml) treatments at 4 mg/ml.
Figure 2: Effects of the fermented extract of Achyranthes japonica Nakai (FAJ) on cell viability (A), nitric oxide (NO) (B) and prostaglandin E2 (PGE2) production (C) in LPS-induced RAW 264.7 cells. All data are expressed as mean ± S.D (n=3). ###P<0.001 compared with the control group; *P<0.05 compared with the LPStreated group.
Effects of AJ extracts on pro-inflammatory cytokine production
To investigate the inhibitory activities of AJ extracts on the production of pro-inflammatory cytokines, the expression of TNF-α was measured (Figure 3). TNF-α expression was remarkably increased with LPS stimulation (###P<0.001) compared with the normal cells (non LPS). The overexpression of TNF-α was significantly reduced by FAJ treatment (***P<0.05) with different concentrations (1, 2 and 4 mg/ ml), whereas NFAJ extract did not produce any noticeable effect on TNF-α expression.
Figure 3: Effects of fermented extract of Achyranthes japonica Nakai (FAJ) on the mRNA levels of TNF-α in LPS-induced RAW 264.7 cells. All data were expressed as means ± S.D (n=3). The obtained ratio of TNF-α/GAPDH was quantified by using ImageJ software. ###P<0.001 compared with the control group; ***P<0.001 compared with the LPS-treated group.
Effects of AJ extracts on the LPS-induced activation of MAPKs
It is generally accepted that MAPKs are the key signaling pathways that regulate cellular processes, including the release of proinflammatory cytokines under chronic inflammation [11]. The expression of MAPKs (p38, ERK, and JNK) in LPS-induced RAW 264.7 cells were analysed by western blotting. These inflammatory mediators were highly activated with LPS stimulation (Figure 4A-4C). The FAJ extract reduced the expression of pp38 at different concentrations (1, 2, and 4 mg/ml; Figure 4A). The overexpression of pERK, which is linked to the increase of TNF-α production was substantially attenuated by FAJ treatment (Figure 4B). However, FAJ did not have any significant effect on pJNK phosphorylation (Figure 4C).
The anti-inflammatory effects of fermented AJ have been mentioned previously [16]. However, the earlier reports did not elucidate the molecular mechanisms, such as MAPK signaling, which directly regulate the inflammation process. This study found that the fermentation of AJ extracts markedly improved its antioxidant and anti-inflammatory functions, with regards to the scavenging of free radicals and the inhibition of pro-inflammatory cytokines and MAPK pathways.
Polyphenols are the main antioxidant compounds found in plants [29]. Fermentation of the AJ extracts resulted in the increase of total phenolic and flavonoid contents were, indicating that the major polyphenolic compounds, such as 2-hydroxyecdysone and saponin in the extract might increase during the fermentation process. This result is in accordance with a previous report, where the content of 2- hydroxyecdysone increased in AJ after fermentation [16].
The synthetic free radicals, such as DPPH and ABTS+ are widely used tools to determine the hydrogen donation (H+) capacity of antioxidants to toxic free radicals in cells [30]. Nitrite is a highly reactive substance, the ionic form of which increases the risk of chronic inflammatory diseases through cell damage and mutagenesis [31]. FAJ, in this study, demonstrated strong free radical scavenging activity against DPPH, ABTS+, and nitrite, which can be attributed to its high polyphenol content. Perera et al. (2016) also reported that the amounts of polyphenols and the inhibition of free radical scavenging activity of a natural extract were strongly correlated [32].
Hydroxyl (•HO) radicals can cause DNA damage and can induce mutagenesis, cytotoxicity, and lipid peroxidation etc. [33]. It has been reported that O2- causes aging through oxidative stress [34]. Both extracts showed powerful scavenging activities against OH and O2- radicals. And the stronger free radical scavenging activity of FAJ can be attributed to the enrichment of phenolic content in AJ through the fermentation process.
Macrophages play essential roles in inflammation by producing inflammatory cytokines, mediators, and growth factors [35]. The overproduction of NO and PGE2 by macrophage cells is one of the key factors involved in the development of inflammatory diseases [36]. This study found that FAJ was more efficient than NFAJ in inhibiting NO and PGE2 in LPS-induced macrophage cells. An earlier study also suggested that fermented AJ showed very high level of inhibitory effects against NO and PGE2in LPS-induced macrophage cells [16]. TNF-α is a pro-inflammatory cytokine, mainly produced by the nuclear translocation of NF-κB in chronic diseases [9]. FAJ suppressed the mRNA level of TNF-α in LPS-stimulated macrophage cells in a dose-dependent manner (Figure 3). This result is similar to an animal study, where fermented AJ substantially inhibited the levels of TNF-α, IL-4 in rats [16].
Furthermore, FAJ strongly inhibited the activation of MAPK pathways in LPS-induced macrophage cells. It is well-known that the activation of MAPKs, such as pp38, pERK, and pJNK is associated with chronic inflammation [37]. Previous studies have shown that MAPKs accelerated the release and activation of pro-inflammatory cytokines and mediators [38,39]. Therefore, these signaling pathways are important targets for the development of therapeutic agents against inflammation. In this study, FAJ markedly decreased the protein levels of pp38 and pERK macrophage cells, indicating FAJ’s potential as antiinflammatory agent (Figure 4).
Fermentation increased the total phenolic and flavonoid contents in the AJ extract. The increase of polyphenols in FAJ resulted in stronger antioxidative and anti-inflammatory functions. The major effect of fermentation of AJ was its strong inhibitory activity against MAPK pathway, which regulates the production and activation of inflammatory cytokines and mediators. Moreover, FAJ has shown substantial effect in decreasing the production of NO and the expression of TNF-α. Therefore, FAJ could be a suitable candidate as a biomedicine for oxidative stress and chronic inflammation. However, further studies are needed to determine the compositional changes in AJ during fermentation and to corroborate current findings.