Research Article - (2016) Volume 4, Issue 6
The disintegration of orange peel waste in deep eutectic solvents and diluted organic acids is presented in this work. The albedo and flavedo layers of the peel were studied separately, showing faster disintegration of the latter. Addition of water to the deep eutectic solvents lowered the amount of remaining solids and improved the disintegration times. These improvements are subscribed to a decrease in viscosity upon deep eutectic solvent dilution. Each of the individual deep eutectic solvent components were diluted and subjected to the same disintegration tests. The corresponding diluted organic acids showed similar orange peel disintegration performance as the tested deep eutectic solvents, whereas dilutions of the other counterparts did not show any activity. Hence, the active deep eutectic solvent components during orange peel treatment are considered to be their organic acids. Flavonoids and essential oils were released during the treatment, offering new opportunities for the development of orange peel waste valorisation routes.
Keywords: Deep eutectic solvents; Orange peel; Food waste; Disintegration
Waste is a term that is quickly associated with landfills, pollution and domestic garbage. Nowadays, however, waste streams are increasingly regarded as renewable sources for materials and chemicals. Crop residues in particular are globally abundant and potentially rich in added-value functionalised components e.g., fibres, oils, proteins, waxes, dyes, and flavonoids [1-3]. Obtaining residues with constant quality and composition is challenging though, for instance due to decentralised harvesting and seasonal influences.
Food processing facilities could offer a well-defined waste stream, since they have to deliver products that constantly meet quality standards. Orange peels (OPs) are an excellent example of processing residues that contain many potential products [4]. In Table 1 the composition of OPs is shown, highlighting the currently commercially exploited compounds. Roughly 71 Mton of oranges were produced worldwide in 2013 [5]. Approximately 40% of all harvested oranges are treated by the juicing and canning industries, of which 40-50 wt.% ends up as processing peel waste [6].
| Compounds | wt.% (dry weight basis) |
|---|---|
| Soluble sugars [12,28-31] | 10-41 |
| Cellulose [12,28-33] | 9-37 |
| Hemicellulose [28-33] | 6-14 |
| Lignin [12,28-30,32,33] | 1-9 |
| Pectin [28-31] | 14-43 |
| Oils and fat (incl. d-limonene) [28-31] | 2-6 |
| Flavonoid[28,31] | 4-6 |
| Protein [12,28-31,33] | 6-9 |
| Ash [12,28-32] | 2-4 |
Table 1: Composition of orange peels, commercially exploited compounds are highlighted.
Despite its great potential [7], OP waste is often still used as lowvalue animal feed additive [8], digested, incinerated or even dumped [9]. Prevailing OP valorisation processes focus on the subsequent recovery of essential oils (mainly d-limonene) and/or pectin. Essential oils are traditionally recovered by cold pressing, steam/hydrodistillation or solvent extraction. Cold pressing and distillation require high mechanical and thermal energy inputs, respectively. Additionally, the elevated temperatures applied (distillation) and the presence of air at elevated pressures (cold pressing) can alter the chemical composition of the oils [10,11]. Depending on the quality of the remaining solids after essential oil isolation, pectin recovery is performed in series or as a separate process. For pectin production, OP is commonly subjected to a mineral acid hydrolysis step followed by ethanol precipitation [12], causing unwanted salt formation upon waste water neutralization.
Many new techniques have evolved for OP treatment, aiming at decreased energy usage, orange oil/pectin quality improvements and process intensification [4]. Among them are ultrasound [13-15], microwave [14,16], supercritical fluid [17] and ionic liquid [11] (IL) extraction. Ultrasound and microwave processes modify biomass cells, accelerate mass transfer rates and the latter are able to heat samples rapidly and accurately [3,16]. These physical treatments are therefore often used to complement affinity extractions with solvents like ILs [18]. ILs can be roughly defined as (organic) salts that melt below 100°C. By designing combinations of cations and anions, new taskspecific solvents can be synthesized. The ILs that proved to dissolve lignocellulosic biomass [19,20] were also screened for OP processing [11].
Following this strategy, the deep eutectic solvents (DESs) that showed biopolymer dissolution in our previous study [21] were selected for OP treatment in this work, see Table 2. DESs are mixtures of two or more components that exhibit (extreme) eutectic behaviour due to self-association, resulting in a liquid at temperatures below the melting temperatures of its individual components [22]. This allows the utilization of their functional groups at mild temperatures without an additional solvent. In 2004, DESs were presented as an alternative to ILs [23], mainly because they display similar physicochemical properties but are prepared more easily. By mixing the components under mild heating, a clear DES can be formed without further purification.
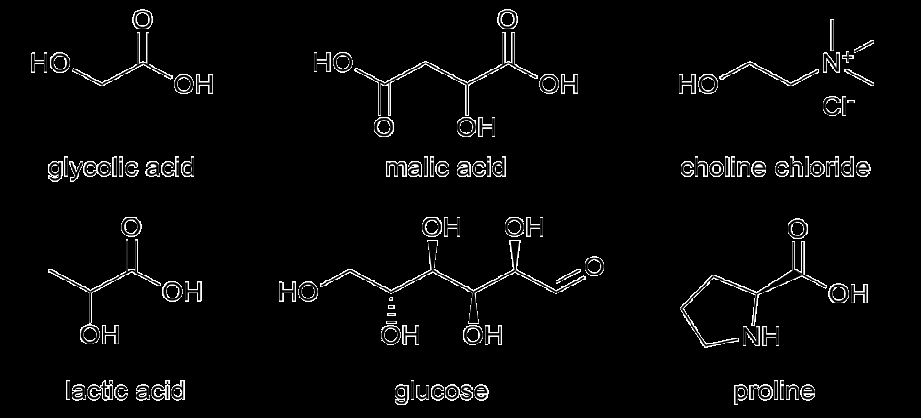 |
|||
|---|---|---|---|
| Component 1 | Component 2 | Ratio | Abbreviation |
| Lactic acid | Choline chloride | 2:1 | LacA:ChCl 2:1 |
| Lactic acid | Proline | 1:1 | LacA: Pro 1:1 |
| Lactic acid | Glucose | 5:1 | LacA:Glc 5:1 |
| Glycolic acid | Choline chloride | 3:1 | GlyA:ChCl 3:1 |
| Malic acid | Choline chloride | 1:1 | MaliA:ChCl 1:1 |
Table 2: An overview of the deep eutectic solvents used for orange peel treatment.
It is likely that the studied DESs show different interactions to each of the components listed in Table 1, hence the structure of OP is of relevance. OPs mainly consist of two layers, the albedo (inner) and flavedo (outer) layer, which account for about 25 and 10 wt.% of the whole fruit, respectively. The albedo is the white spongy cellulose, starch and pectin-rich part, containing most of the flavonoids, amino acids and vitamins [4]. The typical orange colour and odour are provided by the flavedo, embedding the essential oil glands and lignin [4]. Considering the low starch and cellulose and the high lignin solubilities found for the DESs in the aforementioned screening [21], it was expected that flavedo disintegrates more easily than albedo.
Glycolic acid (≥ 99%), choline chloride (≥ 98%) and L-proline (pharmaceutical grade) were purchased at Sigma-Aldrich. DLMalic acid (≥ 99%) and D (+)-glucose (≥ 90%, 8-10% H2O) were acquired from Merck Milipore and crystalline Purac® L-lactic acid (pharmaceutical grade) was kindly provided by Corbion Purac Biochem BV. The chemicals were used without further purification. Deionized water (18.2 MΩ∙cm-1) was obtained from a Millipore Milli-Q® biocell equipped with a Q-grade® column and is hereafter called MilliQ water. Navel oranges were purchased at the local supermarket (Albert Heijn).
DES preparation
An overview of the prepared DESs and their molar ratios is given in Table 3. The DES components were weighed on a Sartorius Extend analytical balance and added to a screw capped glass flask with magnetic stirring bar. The flask was heated in a thermostatic oil bath (IKA ETS-D5 controller, accuracy ± 0.2 K, precision ± 0.1 K) while being stirred at 150 rpm until a clear liquid was obtained. Lactic and glycolic acid based DESs were prepared at 60°C and malic acid based DESs were prepared at 80°C. The water content of the prepared DESs was determined with a 795 KFT Titrino Metrohm Karl Fischer. If the water content exceeded 1 wt.%, the DES was dried under vacuum in a desiccator with silica gel until the water content was below 1 wt.%. The diluted DESs -5, 10 and 20 wt.% of water- were prepared by pipetting water to untreated DESs, correcting for the initial water content.
| Solvent | Peel loading | Peel type | Disintegration | Time (h) |
|---|---|---|---|---|
| GlyA:ChCl 3:1 | 10 wt% | OP | complete | 4 |
| GlyA:ChCl 3:1 | 20 wt% | OP | complete | 5.5 |
| GlyA:ChCl 3:1 | 20 wt% | flavedo | complete | 5 |
| GlyA:ChCl 3:1 | 20 wt% | albedo | partial | 24 |
| GlyA:ChCl 3:1 | 30 wt% | OP | partial | 24 |
| [C2mim]OAca | 20 wt.% | OP | complete | 3 |
| [C2mim]OAca | 30 wt.% | OP | complete | 24 |
| [C2mim]OAca | 50 wt.% | OP | none | 24 |
| [C4mim]Cla | 20 wt.% | OP | partial | 24 |
| [Amim]Cla | 20 wt.% | OP | partial | 24 |
a Data reprinted from Bica et al. [11]
Table 3: Results of the disintegration experiments at 80°C, with orange peel (OP), flavedo and albedo at different peel loadings. The disintegration was considered complete when no intact OP particles could be observed visually.
Orange peel treatment
OP was carefully cut with a scalpel into pieces of approximately 2 × 2 × 2-5 mm. Extra attention was paid while cutting the flavedo in order to maintain the oil glands and prevent the release of essential oil before extraction. The dissolution experiments were performed in 100 mL screw capped flasks or round-bottomed flasks. The total weight of the solution was always 30 g, the peel (fraction) was added to the DES according to the biomass loading. The mixtures of DES and OP were placed in a thermostatic oil bath at either 50 or 80°C (IKA ETS-D5 controller) and stirred with a magnetic stirring bar at 300 rpm. Every 30 minutes the flasks were exposed to an intensive light source to monitor the remaining particle sizes and a photo was taken.
pH measurement
pH measurements were performed with a WTW series Inolab 730 pH-meter equipped with a glass probe SenTix81 (pH 0-14/T 0-100°C/3 mol dm-3 KCl). Before each measurement, the device was calibrated with buffer solutions of pH=4 (WTW 4.00 technical buffer) and pH=1 (Merck Titrisol 1.00 ± 0.02 (20°C)) and the electrode was rinsed with MilliQ water.
Intact OP, separate flavedo, and separate albedo particles (Figure 1) were treated with DESs (water content<1 wt.%). Initially, suspensions with a total weight of 30 g and 20 wt.% peel loading were prepared for the three peel fractions and all selected DESs. No dissolution was observed for any of the tested DESs at 50°C. The temperature was increased to 80°C for GlyA:ChCl 3:1 and MaliA:ChCl 1:1. MaliA:ChCl 1:1 only showed partial disintegration for flavedo, no significant effect was observed for OP and albedo. For GlyA:ChCl 3:1 however, no intact OP or flavedo particles were observed after treatment, while the albedo disintegrated to a much lesser extent (Table 3). This confirms that DESs disintegrate flavedo easier than albedo.
Figure 1: Different types of peel used for DES treatment: orange peel (left), flavedo (middle) and albedo (right). All carefully cut into pieces of approximately 2 × 2 × 2-5 mm. Different types of peel used for DES treatment: orange peel (left), flavedo (middle) and albedo (right). All carefully cut into pieces of approximately 2 × 2 × 2-5 mm.
A possible explanation for the complete and relatively fast disintegration of OP compared to albedo, is that the released components from the flavedo promote carbohydrate hydrolysis. The pH of OP can reach values as low as 3.64 [9]. In order to explore the capacity of GlyA:ChCl 3:1, the peel loading was increased to 30 wt.%. After 24 h, the peel was only partially disintegrated. The treatment reduced the OP particle size significantly, the remaining fibres were swollen and had a pale colour (Figure 2). It is therefore likely that the flavedo has been disintegrated, but part of the albedo endured.
Following Table 3, GlyA:ChCl 3:1 shows resembling disintegration times compared to the ILs that were tested by Bica et al. [11]. For both the DES GlyA:ChCl 3:1 and the IL [C2mim]OAc an increase in peel loading results in a tremendous increment of the treatment time. Although an explanation of this behaviour is not given for the ILs, it was observed that the viscosity of the OP solution increased substantially during the dissolution experiments for the DESs. A likely cause of this viscosity increase is the release of pectin, a gelling agent used for cosmetic and food grade purposes. After the disintegration of 20 wt.% of OP, the resulting mixture behaved like an immobilized gel at room temperature. Lowering the OP loading to 10 wt.% had a positive effect on the disintegration time and the viscosity was decreased significantly. Nonetheless, the DESs exhibited extreme viscosities at ambient temperatures after OP treatment.
In literature, it is suggested that the physicochemical properties of DESs, e.g., viscosity, can be tuned upon water addition [24-26]. Up to water contents of 25 wt.%, the intermolecular hydrogen bonding network between the DESs’ components is still predominant [24,25]. Water solvation of the individual DES components occurs between water contents of 25 and 50 wt.%. The viscosity of DESs typically decreases by one order of magnitude if 25 wt.% of water is added. To overcome the viscosity limitations mentioned previously, it was decided to perform the dissolution experiments with DESs containing 5, 10 and 20 wt.% of water. Dried GlyA:ChCl 3:1 (0.85 wt.% water) has a viscosity of 275 mPa∙s at 25°C, after dilution (20.1 wt.% water) the viscosity dropped to 15.4 mPa∙s at 25°C (method used: Supporting Information 2).
Besides GlyA:ChCl 3:1, the LacA:Glc 5:1 DES was selected, since its components are present in or derivable from the OP and it proved to extract plant metabolites [27]. LacA:Glc 5:1 belongs to a sub-category of DESs – the so-called NADESs – because it is composed of natural components and does not form a liquid at room temperature without water addition [24,27]. The addition of water to the DESs prior to OP treatment accelerates the disintegration process (Table 4). A higher water content also results in a higher pH, since the system becomes more diluted. Figure 3 shows that the disintegration time decreases with increasing pH for both DES systems. This is ascribed to a vast decrease in viscosity and the fact that the systems remain very acidic (pH<1). At initial DES water contents above 25 wt.%, the disintegration time is still improved. Hence, the preservation of the DES’s hydrogen bonding network is not determinant for disintegration of OP.
| Solvent | 5 wt.% | 10 wt.% | 20 wt.% | 50 wt.% |
|---|---|---|---|---|
| GlyA:ChCl 3:1 | n.d. | 2.5 h | 1.5 h | 1 h |
| LacA:Glc 5:1 | 3.5 h | 2.5 h | 2 h | 1 h |
Table 4: The influence of the initial water content of deep eutectic solvents (wt.%) on the orange peel treatment time at which no intact OP particles were observed (h). All experiments were performed at 80°C, with a peel loading of 10 wt.%.
In order to examine if one of the individual components is solely responsible for OP disintegration, water dilutions of glycolic acid, lactic acid, glucose and choline chloride were prepared. For glucose and choline chloride no disintegration was detected after a treatment of 24 h at 80°C. All lactic acid and glycolic acid dilutions disintegrated the OP, resulting in a coloured suspension. For the more dilute systems, only tested for diluted acids, the dissolution time increases with increasing pH. It is assumed that the viscosity is relatively unaffected by further dilution at the presented water contents (all >70 wt.%). Probably, the acidity of the solvent is mainly responsible for the disintegration of the albedo part, in that case, an increased pH lowers the activity of the solvent.
A set of samples was filtered after treatment in order to verify the visual observations of complete disintegration and compare the performance of DES, diluted DES and diluted acid. The results are listed in Table 5 and show that despite the observation of complete disintegration, a significant amount of the added solids is still present after treatment as small fibres. This amount of remaining solids decreases considerably upon initial water addition to the DES. The DES and diluted glycolic acid show similar activities. The initial approach was to use DESs for OP treatment, however diluted organic acids show the same performance at lower concentrations. The lowest amounts of remaining solids are found for the diluted DES batches. This might be partially attributed to the added water that improves the mobility of the salt’s ions, allowing choline chloride to improve the OP disintegration. OP treatment with choline chloride solutions did not lead to disintegration, but lowering the pH by increasing the acid content improved both the disintegration time and decreased the remaining solids. The acid thus seems to act as the active component for OP disintegration. Th e viscosity is likely to be a limiting factor, hence the combination of a relatively low viscosity and high glycolic acid concentration (~49 wt% for the diluted DES) lead to a more complete disintegration.
| Solvent | Solids (wt.%) |
|---|---|
| Diluted glycolic acid (pH=1.45) A | 38.1 |
| Diluted glycolic acid (pH=1.45) B | 39.7 |
| Diluted glycolic acid (pH=1) A | 36.7 |
| Diluted glycolic acid (pH=1) B | 35.4 |
| Diluted GlyA:ChCl 3:1 (20 wt.% water) A | 23.9 |
| Diluted GlyA:ChCl 3:1 (20 wt.% water) B | 24.2 |
| GlyA:ChCl 3:1 A | 34.2 |
| GlyA:ChCl 3:1 B | 35.3 |
Table 5: Remaining solids after OP treatment (Supporting Information 2), expressed in % dry weight after treatment / dry weight added OP (wt.%) for different solvents. Tests were performed in duplo, batch A and B. All experiments were performed at 80°C for 2 h, with a peel loading of 10 wt.% (wet basis).
The limiting step of OP disintegration process is probably the hydrolysis of carbohydrate fibres. This is in line with the previously reported results: lignin [21] and flavedo dissolve relatively readily, while cellulose [21] and albedo require harsher conditions (Table 3). Although these trends can be recognized, a more detailed study to the OP disintegration process is necessary. It might be possible to regulate the product release during OP treatment by tuning the process conditions. After processing the OP at 80°C, both the flavonoid hesperidin and the terpene d-limonene were tentatively detected in the liquid (Supporting Information 2 and 3). These products could be isolated in further separation processes, like liquid-liquid extraction or distillation. Alternatively, the liquefied OP could serve as a cosmetic product, since the DESs can be prepared from generally regarded as safe (GRAS) materials [28-33].
DESs were used to disintegrate OP particles at 80°C. As was expected, the flavedo part of the peel was disintegrated easier than the albedo. An extended study to the influence of process conditions on this behaviour, might open pathways for selective flavedo removal. The viscosity seems to be the most limiting factor in the disintegration process. Diluting the DESs with water prior to the treatment can overcome this limitation. The acid of the DESs appears to be the active component for the disintegration of the albedo part of OPs. The dilutions of the DESs’s corresponding acids were as effective during OP treatment as DESs, hence the incorporation of choline chloride and glucose is debatable. However, a detailed composition analysis of the resulting liquids and remaining solids should be performed to explicate the role of each DES component during the OP disintegration. Additionally, other potential products than the identified hesperidin and d‑limonene might be unveiled, allowing the valorisation of OP through treatment with DESs.
More information on the pH after disintegration, used methodology, and hesperidin and limonene detection can be found in the supporting information.
This work was part of the TKI NCI Kiem program (contract 731.013.111), which is financed by the Netherlands Organisation for Scientific Research (NWO). We gratefully acknowledge additional financial support by ETD&C BV and Corbion Purac Biochem BV for the provision of crystalline Purac® L-lactic acid (pharmaceutical grade). Special thanks to Jaap van Spronsen for his help and fruitful discussions.
The authors declare no competing financial interest.