Research Article - (2018) Volume 6, Issue 5
Keywords: Triterpienoids; H. integrifolia; GC-MS; Natural products; Metabolites
Holoptelea integrifolia (Roxb) Planch (Ulmaceae) consists of 15 genera and 200 species. It is widely distributed in tropical temperate regions of the northern and Indian peninsula to Indo-China and Srilanka [1]. The common vernacular names of the plant in India are Chirabilva, Putigandha (Sanskrit), Papri (Urdu), Karanj, Putikaranj (Oriya) (Government of India) [2]. The plant H. integrifolia has been used traditionally for the treatment of inflammation, gastritis, dyspepsia, colic, intestinal worms, vomiting, wound healing, leprosy, diabetes, hemorrhoids, dysmenorrhoea and rheumatism [3]. Bark and leaves are used as bitter, astringent, thermogenic anti-inflammatory, digestive carminative, laxative, anthelminitic, depurative, repulsive, urinary astringent and in rheumatism [4]. In tribal area the juice squeezed out of mucilaginous boiled bark is used for rheumatic swelling [5].
Decoction of the bark used as oxytocic in pregnant ladies, paste of the stem bark is externally used to treat the inflammation of lymph glands, common fever and ring worm and seabies. Decoction of the leaves is used to regulate fat metabolism, treat eczema and coetaneous diseases [6].
Stem bark acts as an anti-inflammatory agent specifically for eyes. Bark and leaf paste of the plant are used externally on the white patches or leucoderma [7].
Bark paste is used for treatment of herpes simplex infection [8,9]. It is also reported for the treatment of intestinal cancer [10].
Bark and leaves of the plants are also used to treat cold, cough and weakness in cattle [11]. Seeds used topically on ring worm and dried fruit is used in polyurea and urinary disorders [12]. Various parts of the tree has been found to be useful in the treatment of bronchitis and obesity [13].
The ethanol crude extracts of stem bark of H. integrifolia was found anti-oxidant which is comparable to the standard vitamin E [14]. A number of 1,4-naphthalenedione (a phytochemical of the plant) derivatives have been found to have anti-bacterial activity [15].
Benzene, chloroform, methanol and aqueous extracts of stem bark of H. integrifolia showed anthelmintic activity against adult earth worm Pheretima posthuman [16].
The tree has been reported to contain various chemical constituents like terpenoids, saponins, tannins, proteins, carbohydrates, flavanoids and alkanoids [1].
The stem bark contains the triterpenoidal-fatty acid esters, holptelin-A (epi-friedelinol palmitate) and holoptelin-B (epifriedelinol stearate), friedelin and epi-friedelinol, 2- aminonaphthaquinone and β-sitosterol, β-D-glucose were also isolated from stem bark [17].
Fractionation of crude methanol extract of the stem bark of H. integrifolia lead to the isolation of lupeol, ellagic acid and β-sitosterol- D-glucoside [18]. Lupane series compounds, betulinic acid and betulin, were also reported in the ethanolic extract of bark of H. integrifolia [19].
Plant materials and chemicals
The bark of the Holoptelea integrifolia plant was collected from the premises of the University of Karachi, Pakistan during January-April 2014 and identified by Prof. Dr. Ghazal Hafeez Rizwani Department of Pharmacognosy, University of Karachi. A herbarium voucher specimen No. 0045 was deposited in the museum of the Department of Pharmacognosy at University of Karachi, Pakistan [20]. The purified chemicals and all other reagents used during the research work were commercially purchased from Merck (Germany).
Fractionation and isolation
Holoptelea integrifolia plant bark (10 kg) were cleaned and then chopped into small pieces. These pieces were percolated in 80% methanol at room temperature for 15 days. The percolate was filtered thrice separately by using Whatman filter paper No.1. Thereafter, under reduced pressure and controlled temperature 40°C, the filtrate was evaporated to dryness and the methanolic extract was lyophilized to a powdered form. Lyophilized powder (300 g) was partitioned with an equal quantity of distilled water (450 ml) and ethylacetate (450 ml) 1:1, ethylacetate layer was evaporated under reduced pressure and temperature 40°C to obtain ethylacetate extract [20]. The ethylacetate extract was triturated with warm n-hexane to obtain n-hexane soluble fraction (HIB-EA-HS).
GC-MS for identification of the constituents
n-hexane soluble fraction (HIB-EA-HS) was subjected to GC-MS to identify the compounds. For gas chromatography-mass spectrometric detection (GC-MS) a Hewlett Packed 5890 gas chromatograph was combined with a Jeol, JMS-600 mass spectrometer operating in EI mode with ion source at 250°C and electron energy at 70 eV was used. Injector was set at 260°C with splitting ratio 1:30. Mass spectral survey was performed using MS libraries.
It is important to mention that the GC-MS and EIMS analyses were performed from an outsource laboratory (H.E.J Research Institute of Chemistry, University of Karachi, Karachi, Pakistan) (Tables 1 and 2).
| Peak # | Scan # | R.T | M. Formula | M.Wt | Width | Area | Area Sum | Area Sum % |
|---|---|---|---|---|---|---|---|---|
| 22 | 10410 | 80.307 | C29H50O | 414 | 0.056 | 9174961 | 357689120 | 2.5650 |
| 23 | 10427 | 80.44 | C30H50 | 410 | 0.065 | 7146787 | 357689120 | 1.9980 |
| 24 | 10481 | 80.824 | C30H50O2 | 442 | 0.077 | 5157798 | 357689120 | 1.4419 |
| 25 | 10521 | 81.128 | C30H48O | 424 | 0.077 | 21693201 | 357689120 | 6.0648 |
| 26 | 10575 | 81.521 | C30H50O | 426 | 0.069 | 16576489 | 357689120 | 4.6343 |
| 27 | 10606 | 81.744 | C29H46O | 410 | 0.06 | 4860629 | 357689120 | 1.3588 |
| 28 | 10628 | 81.906 | C29H48O | 412 | 0.06 | 3049125 | 357689120 | 0.8524 |
| 29 | 10699 | 82.421 | C29H48O | 412 | 0.075 | 20061002 | 357689120 | 5.6085 |
| 30 | 10773 | 82.957 | C30H50 | 410 | 0.08 | 22764171 | 357689120 | 6.3642 |
| 31 | 10832 | 83.404 | C30H52O | 428 | 0.083 | 51776991 | 357689120 | 14.4754 |
| 32 | - | 83.889 | - | - | - | - | - | - |
| 33 | 10953 | 84.277 | C32H54O4 | 502 | 0.086 | 3527380 | 357689120 | 0.9861 |
| 34 | 11020 | 84.775 | C42H64O2 | 600 | 0.094 | 4083672 | 357689120 | 1.1416 |
| 35 | 11129 | 85.577 | C30H52O | 428 | 0.111 | 18818976 | 357689120 | 5.2612 |
Table 1: Data obtained by GC-MS of the fraction (HIB-EA-HS).
| Peak # | Scan # | Fragment ions m/z |
|---|---|---|
| 22 | 10410 | 414 (80), 396 (53), 381(40), 367(5), 355 (13), 329 (45), 303 (45), 255 (42), 231 (25), 213(45), 145 (70), 107 (75), 81 (78), 55 (100). |
| 23 | 10427 | 410 (50), 395 (30), 381 (100), 355 (10), 327 (9), 303 (8), 281 (50), 269 (25), 253 (22), 218 (75), 207 (95), 161 (35), 145 (58), 121 (60), 105 (52), 95 (75), 69 (75), 55 (98). |
| 24 | 10481 | 442(5), 411(10), 363(10), 337(4), 313(4), 288(6), 245(8), 234(25), 203(60), 189(100), 175(50), 147(45), 135(75), 95(80), 81(75), 69(55), 55(55). |
| 25 | 10521 | 424 (12), 355 (2), 313 (2), 281 (20), 253 (5), 231 (8), 218 (100), 203 (25), 147 (22), 135 (25), 95 (27), 81 (27), 55 (37). |
| 26 | 10575 | 426 (10) , 297 (2), 231 (2), 218 (100), 207 (28), 147 (20), 95 (26), 55 (26). |
| 27 | 10606 | 410 (53), 395 (6), 355(8), 297 (3), 253 (15), 227 (8), 187 (28), 174 (100), 161 (40), 55 (40). |
| 28 | 10628 | 566 (2), 523 (2), 466 (2), 455 (2), 430 (12), 408 (19), 355 (8), 327 (7), 270 (53), 227 (8), 207 (100), 175 (30), 147 (35), 133 (47), 105 (35), 95 (55), 83 (70), 69 (72), 55 (98). |
| 29 | 10699 | 412 (30), 397(10), 370 (20), 355(12), 327 (12), 289 (21), 271(12), 229 (48), 187 (13), 147 (28), 124 (100), 95 (38), 69 (35), 55 (45). |
| 30 | 10773 | 566 (2), 523 (2), 503 (2), 479 (2), 430 (2), 410 (100), 395 (22), 355 (4), 327 (4), 297 (4), 269 (45), 256 (7), 227 (12), 207 (53), 187 (20), 174 (50), 160 (55), 147 (50), 136 (100),107 (55), 95 (75), 81 (70), 69 (53), 55 (80). |
| 31 | 10832 | 428 (10), 413 (15), 395(8), 275 (24), 231 (25), 207 (30), 165 (65), 135 (35), 109 (75), 95 (100), 69 (80), 55 (65). |
| 32 | Not scanned | |
| 33 | 10953 | 523 (2), 479 (2), 426 (5), 383 (10), 355 (10), 313 (10), 281 (48), 253 (20), 229 (8), 207 (100), 191 (20), 133 (30), 107(45), 95 (52), 81 (60), 55 (98). |
| 34 | 11020 | 523 (2), 504 (2), 479 (2), 454 (2), 426 (5), 406 (22), 394 (10), 355 (10), 327 (8), 281 (48), 254 (28), 227 (5), 207 (100), 193 (32), 165 (22),147 (23), 135 (27), 121 (24), 107 (27), 95 (32), 81 (37), 69 (53), 55 (55). |
| 35 | 11129 | 428 (80) , 398 (20), 355 (10), 331 (14), 287(30), 281 (28), 245 (54), 231 (25), 207 (75), 191 (25), 161 (23),147 (28), 137 (68), 107 (52), 95 (52), 81 (67), 55 (100). |
Table 2: Data obtained by GC-EI-MS of the fraction (HIB-EA-HS).
Triterpenes were obtained using GC-MS using n-hexane soluble fraction (HIB-EA-HS).
Triterpenes are rarely identified through GC-MS. These large molecules are usually non-volatiles and elute later from columns at elevated temperature. Therefore, if any, usually undergo thermal degradation some triterpenes or triterpenoids can survive thermal degradation and thus the current study has resulted in the identification of eight triterpenoids, thermally stable under the analytical conditions.
Isolation of β-sitosterol and friedelan-3-ol in earlier studies from bark of H. integrifolia [17] were found helpful in confirming the mass spectrum of peak #22 (scan 10410) and peak #31 (scan 10832) as β- sitosterol (1) and friedelan-3-ol (7) respectively. Betulin has been reported from the bark of the plant [19] therefore peak #24 (scan 10481) was confirmed as betulin (3) from its previous GC-MS data and then also confirmed by comparing the data with electronic mass spectral library.
β-sitosterol (1) is known to possess anti-inflammatory and antipyretic [21], and antioxidant, and anti-diabetic potentials [22]. It has also been found to exhibit tyrosinase inhibitory [23] and radical scavenging properties [24]. It is a proven anticancer against certain cancers, human cancer cell lines (HL-60) [25]. Anticancer activity of compound (3) has been reported from the ethanolic extract of the bark of H. integrifolia [19].
The mass spectrum of peak #23 (scan 10427) and peak #25 (scan 10521) showed the molecular peak [M]+ at m/z 410, 424 respectively. Two characteristic ion peaks at m/z 218 and 207 were observed for peak #23 (scan 10427) and for peak #25 (scan 10521) at m/z 218 and 203. All these ion peaks resulted from retro-Diels-Alder cleavage of ring [26]. For peak #23 (scan 10427) other fragment ions were also observed at m/z 410 [M-CH3]+ and 395 [M-2CH3]+. This fragmentation pattern when compared with electronic mass spectral library confirmed the presence of olean-13(18)-ene (2) and α- amyrenone (4). Diol of olean-13(18)-ene (caflodiol) has been reported as anti-tumor, and its cytotoxic activity against human cancer cell lines [27]. Anti-Inflammatory and Antihypersensitivity effect of α- amyrenone (4) has been documented [28].
For peak #27 (scan 10606) strong intensity molecular peak [M]+ was observed at m/z 410. Loss of side chain from C-17 gave characteristic fragment ion peak [M-141]+ at m/z 269 but it was observed with one methyl group less at m/z 253. Two other very typical fragment ions of cholesta-3, 5-dien-7-one moiety were identified at m/z 187 and 174 (base peak). Both of these peaks arise due to the cleavage of ring C [29]. These findings upon comparison with electronic mass spectral library, were suggestive of the spectrum to be of stigmasta-3, 5- diene-7-one (5). Compound (5) has been identified from the stem bark of P. angolensis by GC-MS analysis [30]. It has been reported as a free radical scavenge [31].
In case of peak #29 (scan 10699) the mass spectral data showed molecular ion peak [M]+ with m/z 412, with other peaks of fragment ions at m/z 397, 370, 355, 271, 147 and 124. The molecular ion peak 412 represents the intact molecule and also gives the exact molecular weight of the compound. The m/z 397 is associated with the loss in mass of 15 from the molecular ion. This can be associated with the loss of methyl (CH3) group [M-CH3]+. The m/z 370 arises from the loss in mass of 29. This is associated with the loss of ethyl group (C2H5) from m/z 397. The m/z 355 resulted from loss in mass of 15(CH3) from m/z 370. The m/z 271 resulted from loss in mass of 141 (C10H21) from the molecular ion. This gave vital information about the presence of a bulky side chain in the molecule. This is a characteristic of steroidal fragmentation pattern. The m/z 147 and 124 confirms the presence of rings and it resulted from the decomposition of m/z 271 [32]. This fragmentation pattern when compared with electronic mass spectral library confirmed the presence of stigmast-4-en-3-one (6). Compound (6) has been isolated as a constituent from the bark of Anacardium occidentale (Cashew) with its hypoglycaemic activity [33].
Peak #35 (scan 11129) showed strong molecular ion peak [M]+ at m/z 428. Other fragment ions were observed at m/z 398 [M-2CH3]+, 287 [M-side chain]+ and due to ring cleavage at m/z 207. The fragmentation pattern when compared with electronic mass spectral library confirmed the presence of citrost-7-en-3ol (8). It has been reported with its antioxidant property in the literature [34].
Identified and unidentified compounds (UI) were listed in Table 3 by following the same order of compounds as mentioned in result.
| Peak # | Scan # | R.T | M. Formula | M. Wt | Name of Compound (number) | ||
| 22 | 10410 | 80.307 | C29H50O | 414 | β-Sitosterol (1) | ||
| 23 | 10427 | 80.44 | C30H50 | 410 | Olean-13(18)-ene (2) | ||
| 24 | 10481 | 80.824 | C30H50O2 | 442 | Betulin (3) | ||
| 25 | 10521 | 81.128 | C30H48O | 424 | α-Amyrenone (4) | ||
| 26 | 10575 | 81.521 | C30H50O | 426 | UI-1 | ||
| 27 | 10606 | 81.744 | C29H46O | 410 | Stigmasta-3, 5-diene-7-one (5) | ||
| 28 | 10628 | 81.906 | C29H48O | 412 | UI-2 | ||
| 29 | 10699 | 82.421 | C29H48O | 412 | Stigmast-4-en-3-one (6) | ||
| 30 | 10773 | 82.957 | C30H50 | 410 | UI-3 | ||
| 31 | 10832 | 83.404 | C30H52O | 428 | Friedelan-3-ol (7) | ||
| 32 | Not scanned | Not scanned | |||||
| 33 | 10953 | 84.277 | C32H54O4 | 502 | UI-4 | ||
| 34 | 11020 | 84.775 | C42H64O2 | 600 | UI-5 | ||
| 35 | 11129 | 85.577 | C30H52O | 428 | Citrost-7-en-3ol (8) | ||
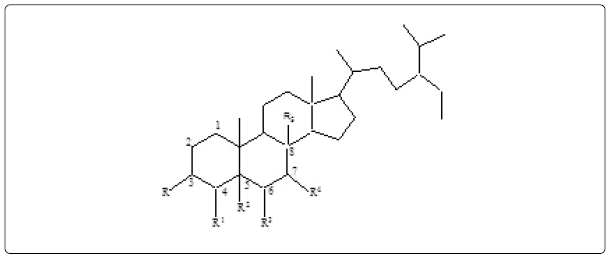 R=OH, R1=H, R2=R3=Δ, R4=H, R5=H, ß-Sitosterol (1) R=O, R1=R2=Δ, R3=H, R4=H, R5=H, Stigmast-4-en-3-one (5) R=R1=Δ, R2=R3=Δ, R4=O, R5=H, Stigmasta-3,5-diene-7-one (6) R=OH, R1=CH3, R2=H, R3=H, R4=R5= Δ, Citrost-7-en-3-ol (8) |
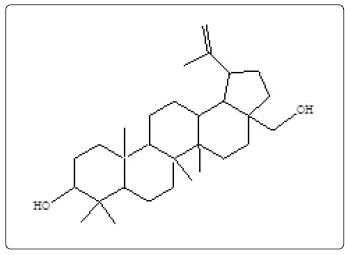 Butelin (3) |
||||||
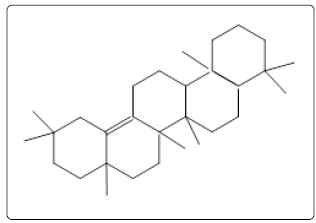 Olean-13(18)-ene (2) Olean-13(18)-ene (2) |
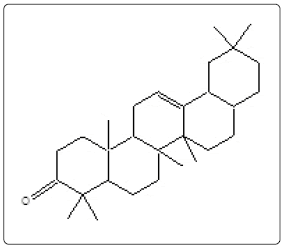 α-Amyrenone (4) |
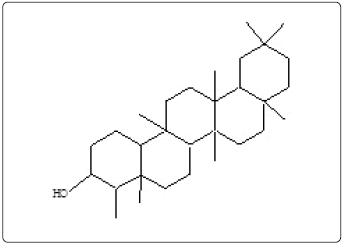 Friedelan-3-ol (7) |
|||||
Table 3: Compounds identified through GC-MS.
Identified triterpenoids (1-8) have also been identified and isolated from different plants but only one or two constituents were found in each species. In bark extract of Holoptelea integrifolia (Roxb.) Planch they all are gathered. Most of them have anticancer activity, and some have antioxidant, hypoglycemic, anti-inflammatory activity. Therefore, bark of H. integrifolia is rich with medicinal constituents and further study may lead to isolate an anticancer bioactive compound.