Research Article - (2017) Volume 5, Issue 7
In this work, the study of the composition of Pistacia lentiscus volatile fraction in El Kala region is targeted. The chromatographic analyses (CPG and CG-SM) of the Pistacia lentiscus extracts allowed to identify solvent, that is the hexane, thirty components, in which three of them are considered major 2-Methoxy,4-vinlphenole (36.5%), 2,3- dihydrobenzofuran (25.50%). and phenyl ethanol (10.85%) and thirty-six other components by the polar solvent with the DMSO as a major one, the oleamide (26.07%).
Keywords: Volatile fraction; CPG; CG-SM; Pistacia lentiscus
The Pistacia lentiscus is a shrub that can reach three meters of height that finds its roots in places like scrublands this dioecious plant belongs to the family of anacardium [1] and is distinguished by foliage compared to other pistachio trees. Several tests have been carried out on the oil extracted from the ripe fruit proving that it contains unsaturated and saturated fatty acids and vitamin [2,3]. However, our bibliographic research has shown that no study has been carried out on the basis of the determination of volatile scopes. We are interested in warring out this work to determine the chemical constituents of volatile fractions with the help of the CPG-MS technical.
Materials and vegetal
The conditions for harvesting the fruit are summarized in the Table 1.
| Botanical Name | Date harvested | lieu | Development Stage | season | Middlevegetative |
|---|---|---|---|---|---|
| Pistacialentiscus | November2016 | Oued Hout | Fruit ripe | rain | Forest |
Table 1: Summary of harvest conditions.
The fruits are washed with distilled water to remove organisms responsible of the deterioration of their quality then they are immersed in nitrogen liquid for chemical and physical stabilization. The fruits are dried in an oven at 95º C for 8 hours and then ground with a coffee grinder to obtain a fine powder [3-5].
Volatile extracts preparation
The extraction of the volatile fraction on 160 g of powder has been realized by hydro distillation, using an apparatus of (Clevenger 1928) type, for 3 hours, in a liter of water within a balloon of 2 liters [6,7]. The balloon is surmounted by a column of 60 cm length, related in its turn to a refrigerant followed by liquid extraction-hydrolat liquidusing the hexane and the DMSO as solvents. The extraction output of these substances is less than 0.4%.
Chromatographic analysis
The analyses were realized with a chromatograph (Agilent technologies 6890) enhanced with a flame ionization detector (FID) of a capillary column HPS (30 m × 0,32 mm) film thickness 0.25 mm) the helium is the vector gas. The injector temperature is 270º C, and des detector one is 250º C. The furnace temperature program consists of one isotherm at 80º C/min, followed by a temperature ramp of 5º C/min up to 310º C (2 min) [8]. The injection is made by mode split less. The volume injected is 1 μl. The components identification was executed drawing on their indices of KOVATS (IK) and on gas phase coupled to mass spectrometry (CG-SM). The latter was made on a gas phase chromatograph of Agilent Technologies 6890 type, coupled to mass spectrometer Autospee M-610. The ionization mode is the electronic impact of 70 eV and the detection is made by an HRMS analyzer (high resolution mass spectrometry) type E-B-E within the masses range of 50-800 Da [9].
The temperature programming is identical to the one used previously for the detection by FID and the injection is by mode split less. The apparatus is related to data processing system managing a mass spectral library NIST 98 [10,11]. The components identification is based on the comparison of their mass spectra (CPG-SM) respective to library spectra (NIST 98) and (Adams 1995) library. Ans also based on the indices calculation of Kovats, these indices of Kovats were calculated following the equation 1 after an analysis under the same conditions of the chromatography of alkane series.
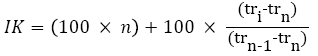
Tri=retention time of the volatile fraction compound
Trn=retention time of the alkane at n carbon
Trn-1=retention time of the next alkane
The results obtained (Table 2) by solvent hexane and DMSO show that there is a great diversity of the chemical constituents of qualitative and quantitative aspects of the composition of the volatile liquor contains alcohols, aldehydes, ketones, nitrogen compound and furanic derivatives.
| Compound | RI | Hexane (%) | DMSO (%) |
|---|---|---|---|
| 2-Ethylhexanol | 1038 | 1.45 | 1.28 |
| Eucalyptole | 1045 | 1.10 | - |
| 2,3 dihydrolenzofurane | 1050 | 25.50 | - |
| Phényléthanal | 1053 | 10.85 | 13.28 |
| 1 propylbenzene | 1056 | - | 10.02 |
| 2-methyoxyphénol | 1085 | 2.07 | 0.03 |
| methyl benzoyl formate | 1073 | - | 0.22 |
| O-methoxyphenylacétate | 1095 | - | 0.07 |
| Thozone | 1102 | 0.48 | - |
| 2,6 dimithyleyclohexanol | 1107 | - | 0.75 |
| 2-indanone | 1150 | 0.56 | - |
| 3-Phenylprop 2-énal | 1157 | - | 1.30 |
| Bornéol | 1162 | 1.39 | 0.65 |
| Terpinen-4-ol | 1173 | - | 0.16 |
| m-methylacétophone | 1181 | - | 0.12 |
| a-terpinol | 1186 | 1.50 | - |
| 2,2’ dibutoxyéthylether | 1189 | - | 3.88 |
| Verbenone | 1206 | 0.34 | 1.27 |
| Benzothiazole | 1120 | 1.90 | 2.65 |
| 2,5 diméthyle3 enylformate | 1226 | 0.40 | - |
| 2-butylcyclohexanone none | 1260 | 0.071 | - |
| 2 propylpiperidine | 1250 | - | 0.02 |
| Thymol | 1300 | 1.84 | - |
| 2 methoxy-4-vinylphénol | 1310 | 36.51 | - |
| 4-hydroxy 2-methylacitophone | 1311 | - | 15.60 |
| 1,4 diéthoxy-benzené | 1341 | - | 0.58 |
| Eugénol | 1353 | 0.75 | 0.18 |
| gdicalactone | 1358 | 0.30 | 0.45 |
| 3,5,5 trimethyhexan 1 | 1364 | 0.25 | - |
| Vanilline | 1395 | - | 3.16 |
| g Cadinene | 1422 | - | 0.06 |
| Esterméthylique de l’acide 9 oxononanoique | 1430 | - | 0.29 |
| bionome | 1480 | - | 0.17 |
| bcubebene | 1475 | - | 0.51 |
| 4,6 de tert butyl 2 méthylphénol | 1506 | 0.28 | 0.88 |
| Benzophénone | 1619 | 0.14 | 0.21 |
| T murolol | 1635 | - | 0.16 |
| acadinol | 1645 | - | 0.30 |
| 3,5 di-tertbutyl 4 hydrobenzaldchyde | 1556 | 0.35 | 0.65 |
| Icosanol | 1786 | - | 0.10 |
| 2,6,10,15 tetraméthylheptadécane | 1791 | - | 0.42 |
| Tetradicanamide | 1814 | - | 0.01 |
| 13 epimanol | 2042 | 0.02 | - |
| Linolèate de methyle | 2086 | - | 1.98 |
| Isotréatate de méthyle | 2119 | - | 0.20 |
| Déamide | 2351 | 0.13 | 26.07 |
| Squatène | 2812 | 0.59 | - |
| Isopropyl palmitate | 2018 | 0.21 | - |
| Nom identifie | 1266 | 0.32 | - |
| Indolizine | 1290 | 0.32 | - |
| Non identifiée | 1338 | 0.49 | - |
| Diheptylèther | 1041 | - | 0.98 |
| Camphre | 1140 | 1.17 | - |
| Indolizine | 1290 | 0.32 | - |
| DMSO=Dimethyl sulfoxide | |||
| 1=KOVATS index Relative to alkanes C8-C30 | |||
Table 2: Composition of Pistacia volatile extracts Pistacia lentiscus .
On the one hand, in the hexane extract, thirty components chemical reaction with a predominance of alcohol with 2-methoxyphenol vinyl chloride (36.51) and then the 2,3 dehydrobenzofuran, 25.50% followed of the phenyl ethanol (10.85%) on the other hand the monoterpene compounds and oxygenated monoterpenes are found in large quantities on the other hand the DMSO extract, thirty-six compounds, aldehydes and ketones such as verlanone (1.27%), the range dicatactnoe (0.45%) vanilla (3.16%) and also the alcohols (borneol, eugenol) the terpene compounds are present in small quantities.
The chromatographic analysis of both extracts allowed us to identify the volatile fraction obtained from Pistacia lentiscus oil extract. The detected molecules have different chemical and physical characteristics, alcohols, aldehydes, ketones and nitrogen compounds.
The predominance within the hexane extract fraction is for alcohols, aldehydes and ketones, then are dominant in DMSO extract. Oleamide is a typical component of DMSO extract.
The vanillin is an aroma which has a peach note in DMSO extract with a salient relative rate. When encountering this identified and composed panoply, it is important to assign those that bestow typicality to the fraction volatile aroma.
The information obtained on the various substances could perhaps serve in particular studies on the sensory characterization using CPGolfactometrie which would be an interesting alternative. Forthcoming, this evaluation would serve as a main object of publication in your paper.