Research - (2021) Volume 7, Issue 1
Background: Glycated albumin (GA) and glycated hemoglobin (HbA1c) levels are affected not only by plasma glucose levels but also by albumin metabolism and hemoglobin metabolism, respectively. Our study is aiming to evaluate the role of (GA) as an indicator of the glycemic state and its correlation with HbA1c in hemodialysis patients with diabetes mellitus.
Research Design and Methods: Our study is a cohort prospective study done on 50 chronic uremic diabetic patients 23 males and 27 females at Al-Azhar Assuit University Hospital, from August 2016 to February 2017. All the studied patients were on regular HD 3 times/week, 4 hours session. Multiple regression analysis was done to assess the correlation between glycated albumin and HbA1c as indicators of glycemic state. Patients with pregnancy, nephrotic syndrome, neoplastic disorders, and anemia due to causes other than chronic renal disease as hemolytic anemia, chronic liver diseases, thyroid disorders, and those under immunosuppressive therapy were excluded.
Results: GA had had a highly significant predictor for PG (p-value = 0.001) VS HbA1c%, which was had a significant predictor (p-value = 0.021).
Conclusion: Our study suggested that while HbAlc is considered the gold standard test in estimating the glycemic state in diabetic patients, GA can be used as an indicator of the glycemic state in uremic patients on regular HD.
Albumin; Glycated Hemoglobin A
In diabetes mellitus, glycation of various proteins is known to be increased, and some of these glycated proteins are thought to be involved in the onset and progression of chronic diabetic complications [1]. Of these, HbA1c is widely used clinically as a marker of glycemic control [2, 3]. Because the erythrocyte lifespan is approximately 120 days, HbA1c reflects plasma glucose levels over the preceding 3 months. However, in disorders in which erythrocyte lifespan is shortened, e.g., hemolytic anemia, blood loss, and liver cirrhosis, and with variant hemoglobin’s, measurement of HbA1c is affected, and it does not accurately reflect glycemic control status [4, 5]. KDIGO 2020, demonstrate that, Glycated albumin and fructosamine have been proposed as candidates for alternative long-term glycemic monitoring. These biomarkers reflect glycemia in a briefer timeframe (2–4 weeks) than HbA1c due to their shorter survival time in blood.
In observational studies, glycated albumin is associated with all- cause and cardiovascular mortality in patients treated by chronic hemodialysis. [6]. Because the half-life of serum albumin is shorter than that of erythrocytes, GA reflects plasma glucose levels over a shorter period (approx. 2 weeks). Therefore, in cases of acute changes in glycemic state, GA is more useful than HbA1c as a glycemic control marker [7]. Although GA measurement is not affected by hemoglobin metabolism, it is affected by albumin metabolism [6, 8, 9]. Recently, for diabetic patients with chronic kidney disease undergoing hemodialysis (diabetic nephropathy (DN) stage 5), because of renal anemia low HbA1c levels relative to plasma glucose have been reported [10–12]. In addition, as administered doses of erythropoietin are increased for the patients with renal anemia, HbA1c becomes lower [11, 12]. On the other hand, because GA is not affected by renal anemia, it may be an ideal glycemic control marker for diabetic patients on hemodialysis [10–12].
Patients and methods
The current prospective study including 50 chronic uremic diabetic type 2 patients 23 male and 27 females with age range of (45-61) years who are on regular hemodialysis in Al-Azhar Assuit University Hospital. The study was conducted through the period from August 2016 to February 2017. All patients were on regular HD 3 times/ week, 4 hours session, with hemoflow F6 polysulfone filter, surface area membrane 1.3 sqm, bicarbonate dialysate free of glucose, with minimum Kt /V value of 1.2 as an in Adex of urea removal. Among the patients, those with pregnancy, nephrotic syndrome, neoplastic disorders, anemia due to causes other than the chronic renal disease as hemolytic anemia (e.g., hemoglobinopathies), chronic liver diseases, or thyroid disorders, and those under immunosuppressive therapy were excluded. In order to minimize the effects of diabetic treatments on time-dependent variations of HbA1c and GA levels (Figure 1), we selected patients whose HbA1c levels had been stable for at least the past 3 months: The variations of HbA1c in the past three-monthly determinations were less than 0.5%.
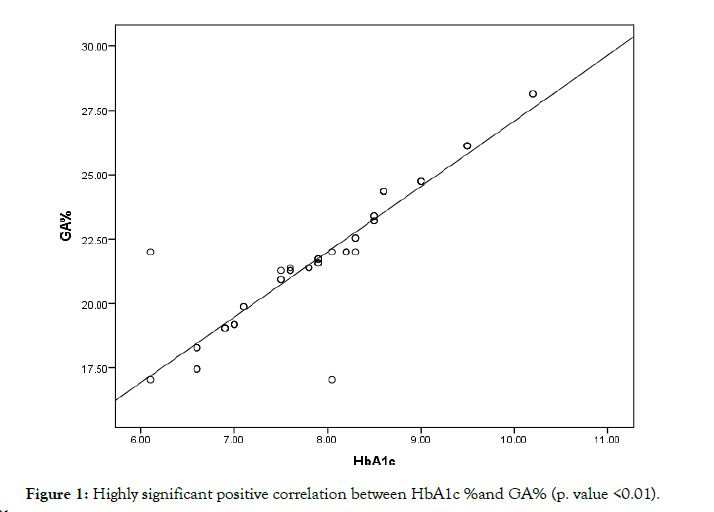
Figure 1: Highly significant positive correlation between HbA1c %and GA% (p. value <0.01).
All the patients were submitted to the following:
Full history including age, sex, duration of DM, drug therapy (insulin, oral hypoglycemic or combined), duration of HD, history suggestive of diabetic complications and other associated illnesses.
• Anthropometric measurements: Bodyweight in Kg and height in meters. Calculating of BMI = BW in kg ÷ (BH in m) 2.
• Thorough general clinical examination: including different body systems.
• Abdominal and pelvic ultrasound.
• Laboratory investigations including CBC, liver and kidney functions tests, 2hrs postprandial blood glucose level, HbA1c, serum albumin, glycated albumin (GA) and GA/HbA1c ratio.
• Serum GA was measured every 3 weeks through 3 months and the mean value of these measurements was estimated and HbAlc was measured once, concomitant with the determination of RBCs, Hb, HCT, total protein, serum albumin, blood urea, and serum creatinine.
• Assay of Glycated Albumin by the following formula:
Glycated Albumin level (gm/dl) X100
GA% = Serum Albumin level (gm/dl)
Assay of Glycated Hemoglobin by Stan bio glycohemoglobin: Quantitative colorimetric determination of glycohemoglobin in whole blood.
The collected data was analyzed using SPSS (Statistical Package for Social Sciences) version 15. Qualitative data were presented as number and percent. Quantitative data were tested for normality by the Kolmogorov-Smirnov test. Normally distributed data were presented as mean ±SD. Pearson's correlation coefficient was used to test the correlation between variables. (P-value >0.05 non- significant, P value< 0.05 significant and P<0.01 highly significant).
The study was conducted on 50 type 2 diabetic patients on regular hemodialysis attending to Al-Azhar Assuit University Hospital, 23 male (46%) and 27 females (54%) with age with a range of (45-61) years and MeanSD (52.5 5.4 years). The anthropometric measures among our patients show weight (kg) Mean SD (78.6 + 9.3), height (cm) Mean SD (170.3 + 3.9), and BMI (kg/m2) Mean SD (27.1 + 3.4). The duration of HD (years) in the studied patients was from 1 to 13 years with Mean SD (4.4 3.3 years). It also shows that the range of duration of DM in the studied patients was from 10 to 23 years with Mean SD (16.6 3.5 years). The laboratory data were demonstrated in the Table 1. In the present study by multiple regression analysis to assess HbA1c and GA as predictors to PG was found that GA Highly significant predictor for PG (p-value = 0.001) VS HbA1c%, which is only significant predictor (p-value = 0.021). The present study shows that, there were a significant negative correlation between BMI and GA% r (-0.24) & p value (0.039*). There is a significant negative correlation between S. Albumin and GA%, (r-0.33) & p value (0.021*). The correlation between PG and HbA1c (Figure 2) & GA% shows a strong positive correlation. p value (0.001**). Also, there is a significant negative correlation between Epo and HbA1c, r value (-0.26). Finally, there is a strong positive correlation between GA% and HbA1c (p value 0.001**) as shown in Table 2.
Table 1: shows the laboratory data of the studied patients.
| Result | ||
|---|---|---|
| Range | Mean + SD | |
| HbA1c% | 6.1 - 10.2 | 7.9 + 1 |
| GA (um/l) | 255.7 – 2964 | 400.1 + 372.6 |
| GA% | 17 - 28.2 | 21.8 + 2.7 |
| Albumin (g/dl) | 3.4 - 4.1 | 3.7 + 0.2 |
| GA/HbA1c ratio | 2.6 - 2.9 | 2.7 + 0.1 |
| PG (mg/dl) | 155 – 265 | 191 ++ 31.7 |
| Hb (g/dl) | 7.9 - 11.6 | 9.9 + 1 |
| Urea (mg/dl) | 125 – 250 | 179.6 + 27.2 |
| Creatinine (mg/dl) | 7 - 11.5 | 8.5 + 1.2 |
| Epo. (iu) | 2000 – 8000 | 4300 + 1568.2 |
Table 2: Shows the Correlation between HbA1c %and GA% versus other variables in the studied patients.
| HbA1c % | GA% | |||
|---|---|---|---|---|
| r | P | r | P | |
| Age | 0.20 | 0.164ns | 0.16 | 0.400ns |
| BMI (kg/m2) | 0.21 | 0.223ns | -0.24 | 0.039* |
| Duration of HD (years) | -0.19 | 0.179ns | -0.15 | 0.288ns |
| Duration of DM (years) | -0.24 | 0.099ns | -0.12 | 0.418ns |
| S. Albumin (g/dl) | 0.24 | 0.201ns | -0.33 | 0.021* |
| Urea (mg/dl) | -0.12 | 0.413ns | -0.18 | 0.223ns |
| Creatinine (mg/dl) | 0.21 | 0.177ns | 0.16 | 0.214ns |
| PG (mg/dl) | 0.84 | 0.001** | 0.87 | 0.001** |
| Epo. (iu) | -0.26 | 0.011** | -0.12 | 0.403ns |
| GA (um/l) | -0.03 | 0.844ns | -0.03 | 0.845ns |
| GA/HbA1c ratio | -0.05 | 0.737ns | 0.11 | 0.444ns |
| GA% | 0.95 | 0.001** | - | - |
* Significant correlation at p<0.05 level
** Highly Significant correlation at p<0.01 level
Ns: non-significant correlation at p>0.05
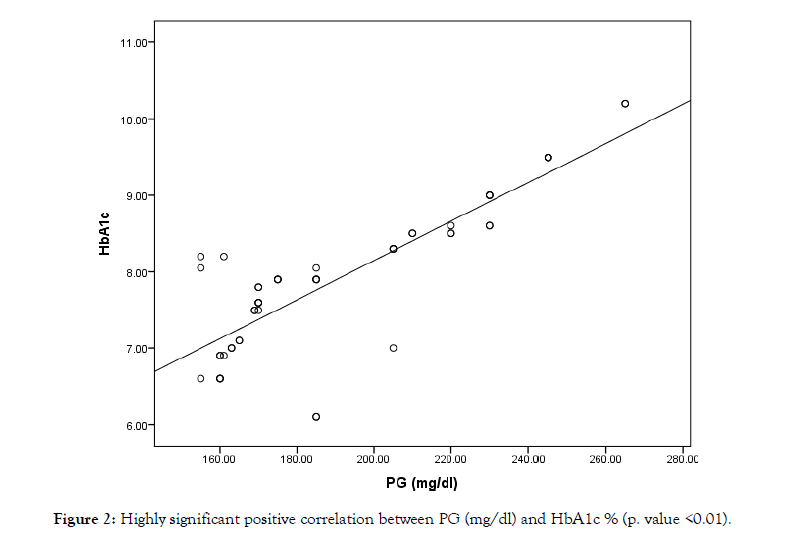
Figure 2: Highly significant positive correlation between PG (mg/dl) and HbA1c % (p. value <0.01).
DM is a metabolic disease with hyperglycemia resulting from defects in insulin secretion, insulin action or both. The chronic hyperglycemia of diabetes is associated with long term damage, dysfunction, and failure of various organs such as nephropathy, retinopathy, angiopathy, and neuropathy. DN now accounts for nearly 50% of incident dialysis patients in the United States [13]. In the advanced stage of renal disease, better glycemic control before HD has been suggested to be associated with reducing the risk of death from diabetic complications. HbAlc has been a cornerstone in the evaluation of dialyzed and non-dialyzed diabetic patients. This measurement relies on a relatively stable RBCs survival, a characteristic typical of the general population but not patients on HD. During HD, the uremic environment, blood loss during treatments, and frequent phlebotomy all contribute to decreased RBCs lifespan. Shortened RBCs survival and red cell transfusions are likely to lower the HbAlc, potentially making it unreliable in assessing glycemic state [14]. Furthermore, it is reported that HbA1c may not reflect the glycemic state in diabetic HD patients. Serum GA was hypothesized to be an alternative marker for the glycemic state in patients with diabetes, which is not affected by changes in the survival time of erythrocytes in the case of type 2 diabetes [15]. In the present study by multiple regression analysis to assess HbA1c and GA as predictors to PG was found that GA Highly significant predictor for PG (p-value = 0.001) VS HbA1c%, which is only significant predictor (p-value = 0.021). A non-significant positive correlation between HbA1c and BMI, while a significant negative correlation between GA and BMI were observed in our study. This was consistent with the findings of Miyashita et al, [16] who showed that, HbA1c % and BMI have a very weak correlation. However, GA % was lower in obese type 2 diabetic patients than non-obese type 2 diabetic patients. Jaw-Kyung et al., [17] reported that, GA % could be affected by various conditions with abnormal metabolism of albumin. Under certain conditions with shortened albumin metabolism, such as hyperthyroidism, nephrotic syndrome, peritoneal dialysis or administered immunosuppressive therapy, serum GA level apparently low, whereas it may be high when albumin metabolism is prolonged, as in liver cirrhosis. However, this was not applicable in our study as the patients were not suffering from any chronic liver diseases which make the relation between GA and serum albumin level insignificant. This finding was in agreement with the finding of Fukuoka et al., [18] who reported that the HbAlc level may inadequately reflect the glycemic state and tend to be underestimated in diabetic patients with end-stage renal disease (ESRD). In contrast, GA is not influenced by Hb, erythrocyte lifespan or EPO therapy. Furthermore, more studies have shown that GA might reflect plasma glucose (PG) levels more correctly than HbAlc, suggesting that GA is a better indicator for PG level compared with HbAlc in ESRD patients with. Also, this finding was in agreement with the finding of Dawlat et al., [19].
Inaba et al., [20], they compared the average PG levels and GA or HbAlc in diabetic HD patients and diabetic patients without chronic kidney disease (CKD) and they found that the degree with which serum GA correlated with PG was identical between the diabetic HD patients and diabetic patients without CKD. The significantly lower level of HbAlc relative to PG and GA (Figure 3) in diabetic HD patients compared with diabetic patients without CKD might propose that the measurement of HbAlc would result in the underestimation of glycemic state in diabetic HD patients. Inaba et al, [20] reported that the measurement of GA% is a more relevant method than HbAlc in assessing glycemic state in HD patients due to the usage of EPO, which is used for renal anemia in around 90% of HD patients, suppresses HbAlc values by 33% on average independent of glycemic state. This not with the agreement with Konya J et al., [21] who found that the clinical usage of EPO increases the proportion of reticulocytes and immature RBCs in circulation with less glycemic exposure time for glycosylation to occur. So, a weak correlation between HbAlc and mean random glucose levels was observed in diabetic patients undergoing HD. Observational data by Cefalu et al., [22] found that, GA is the most accurate predictor of glycemia in diabetic HD patients VS HbA1c which can be used as a predictor of glycemia in diabetic patients, not on HD. Another data by Peacock et al., [12] found that HbAlc levels underestimate glycemic state in HD patients, whereas the percentage of GA relative to the total serum albumin (GA%) accurately reflects glycemic state,
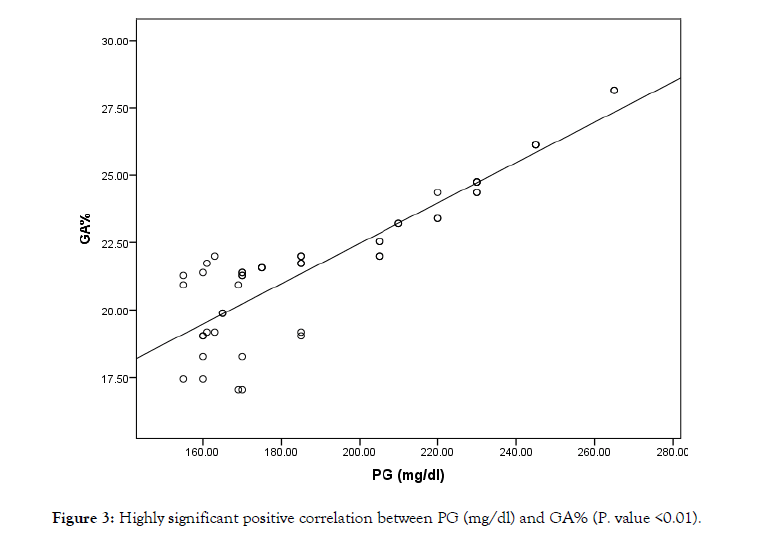
Figure 3: Highly significant positive correlation between PG (mg/dl) and GA% (P. value <0.01).
GA% may be considered to be a useful marker and predictor of the recent changes of the glycemic state in diabetic patients on HD. Our study has some limitation as no control group was included. also, GA was measured more than once and this might had led to changes in patients diet style, so better GA levels.
The authors declare that they have no conflict of interest.
Citation: Azeem H A, Hashim A. M, Alkabeer A. M (2021) The Role of Glycated Albumin as a Marker of Glycemic State in Type 2 Diabetic Patients Under Hemodialysis. J Kidney 7: 201. DOI: 10.35248/2472-1220.21.7.201.
Received: 02-Jan-2021 Published: 25-Jan-2021, DOI: 10.35248/2472-1220.21.7.201
Copyright: © 2021 Azeem H A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.